
Imagine the following: Your doctor prescribes you a medicine and tells you frankly: it does not contain any active ingredients, it is a placebo, but it may help you anyway. Would you believe him/her? And would you take it?
Taking a medical pill when you don’t know whether it contains an ingredient that can improve your symptom or not — this is the situation of patients participating in a drug trial. It is like betting on a horse: you have a 50% chance to win or lose — most bets have much worse chances, however. And you cannot ask your doctor; he/she would claim to be as blind as you. Results (who gets the drug, who gets the placebo) will be disclosed later, after all the patients have completed the study and all data have been evaluated. The only thing you can do is wait and observe what happens to your symptoms and conditions.
The other situation, where a patient might unknowingly receive a placebo is when the doctor feels the patient should not take this sleeping pill, pain reliever, or other medication any longer, e.g. because of concerns of dependency, but the patient feels he cannot do without it. Or when the patient asks for a medicine when the doctor thinks it is not needed but does want to tell, or when the patient insists despite better arguments. In this case, the doctor might prescribe a medication that may not work (e.g. antibiotics in case of a viral infection) or is much less effective than is said.
Both situations provoke some type of ethical concern. In the former case, because your chances of getting effective treatment are less than 100%, which is against the rules set by the Declaration of Helsinki: that every patient should receive the best available treatment for his/her condition, and that doctors should do their very best to relieve the patient’s disease. And it poses an ethical concern in the latter case because the information given (“this is effective”) involves a deception (a lie). Each patient has the right to be fully and correctly informed about the disease and its treatment. While such ethical concerns are not legally binding, they clearly affect the doctor-patient relationship.
When Lee D. Park, M.D. and Uno Covi, M.D. from the Department of Psychiatry at Johns Hopkins University School of Medicine in Baltimore, MD, back in the 1960s, gave their depressed patients placebos, they did exactly this — told them that the pill did not contain an active pharmacological agent (at that time, likely a classical tricyclic antidepressant), but was a placebo. They were surprised to see that it did work (1): 14 of their 15 patients reported improved symptoms a week later, and no difference was found between those that had believed they were taking the placebo and those that had — upon questioning — believed they had received a real drug.
The authors speculated what had brought about this result and compared OLP to psychotherapy, stating, “… two major characteristics of accepted psychotherapeutic techniques were present: on the one hand, support and reassurance were given, while, on the other hand, the responsibility for improvement was thrown back to the patient … the combination of these two elements, support and autonomy, could benefit even a very distressed individual …” (ibd., p 344f). For a long time, this has remained the only example of OLP and has often been seen by sceptics as highly specific for the psychoneurotic patients seen by the two psychiatrists. How wrong they were!
It lasted almost 50 years until two research groups dared to test the OLP paradigm again. In 2008, Sandler & Bodfish from Departments of Pediatrics & Psychiatry, UNC at Chapel Hill, reduced by 50% the actual medication (methylphenidate or amphetamine) in 26 children ages 7-15 with Attention Deficit Hyperactivity Disorder (ADHD) and substituted it with placebos for one week, with full information about the nature of the intervention (2). Such a “dose-extension” study design follows the principles of Pavlovian conditioning (3), one of the (few) basic mechanisms to provoke the placebo effect. Here, the important bottom line is: Reducing ADHD medication and substituting it with placebo was acceptable by children and parents and did not worsen the ADHD symptoms in the eyes of the patients, their parents, and their teachers.
Two years later, a placebo research group at Harvard Medical School, led by Ted Kaptchuk, presented an OLP study in another patient group with irritable bowel syndrome (IBS). This was the first well-designed and effectively-controlled OLP study (4), randomizing 80 patients to placebo treatment (twice daily for 3 weeks) or non-treatment control group to observe the spontaneous variation of symtoms. OLP patients received full disclosure that “… explained in an approximately fifteen minute a priori script the following ´four discussion points´: 1) the placebo effect is powerful, 2) the body can automatically respond to taking placebo pills like Pavlov’s dogs who salivated when they heard a bell, 3) a positive attitude helps but is not necessary, and 4) taking the pills faithfully is critical” (ibd, p 2). Needless to say: OLP produced higher global IBS symptom improvement, less severe IBS symptoms, better adequate relief scores, and trends towards a better quality of life than the control condition.
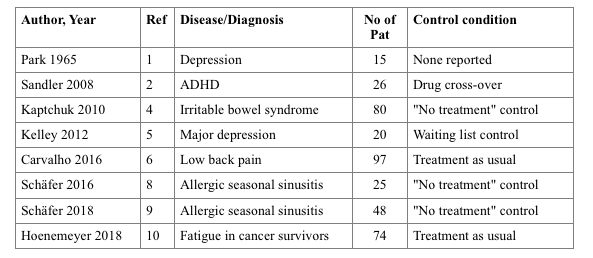
Since then, five more OLP studies have been published, with more clinical conditions: One in depression (5), one in low-back pain (6), two for seasonal sinusitis (7,8), and one for fatigue in cancer therapy (9). They resemble the range of applications, similarities in their strength, but also pitfalls of OLP, as visible in the table: e.g., treatment-as-usual is usually not specified or standardized in the trials, and all study samples are small considering the distribution of the patients on two study arms.
Two experimental studies in healthy controls evaluated different aspects of the OLP paradigm: in one (10), the authors explored whether dosage (one versus two placebo pills per day) affected outcome (it did not), and whether expectancies and adherence contribute to the response (both did). Another experiment (11) tested whether providing a rational for OLP would affect the outcome (placebo analgesia) in comparison with “no rational provided” and a deceptive placebo application. As expected, providing a rational improves the placebo response, but the difference between concealed and open-label application was not significant, questioning the necessity for concealment in placebo administration. This, therefore, may overcome the ethical limitations of placebo application in children (12) and adults (13) much better than with authorized deception (14).
Two meta-analyses have summarized the results so far (15, 16) but missed some important issues that are critical for the validation and implementation of OLP in medical practice. For one, all these examples have shown that while openly-applied placebos affect symptoms (depression, motor activity, pain, fatigue, etc.), none have yet shown that it may also affect disease biomarkers. Secondly, patient (self-)selection has to be tested for biases, e.g., whether the recruited patients are prone to respond to placebo, while others not recruited are not. Last but not least, it would be important to know how OLP compares to effective drug treatment of the same condition and in the same patients. A study protocol of such a four-arm trial (18) has been proposed for treatment of IBS, and we are eagerly awaiting the results.
This is part 3 of a series covering “placebo” provided by Paul Enck and Sibylle Klosterhalfen from the Tübingen University Hospital. Continuous updates on placebo research can be found at www.jips.online.
References:
- Park LC, Covi L. Nonblind placebo trial: An exploration of neurotic patients´ responses to placebo when ist inert content is disclosed. Archives of general psychiatry. 1965;12:36-45.
- Sandler AD, Bodfish JW. Open-label use of placebos in the treatment of ADHD: a pilot study. Child: care, health and development. 2008;34:104-10.
- Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain. 2016;157:1590-8.
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PloS one. 2010;5:e15591.
- Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M. Open-label placebo for major depressive disorder: a pilot randomized controlled trial. Psychotherapy and psychosomatics. 2012;81(5):312-4.
- Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ, Kirsch I. Open-label placebo treatment in chronic low back pain: a randomized controlled trial. Pain. 2016;157:2766-72
- Schaefer M, Harke R, Denke C. Open-Label Placebos Improve Symptoms in Allergic Rhinitis: A Randomized Controlled Trial. Psychotherapy and psychosomatics. 2016;85:373-4.
- Schaefer M, Sahin T, Berstecher B. Why do open-label placebos work? A randomized controlled trial of an open-label placebo induction with and without extended information about the placebo effect in allergic rhinitis. PloS one. 2018;13:e0192758.
- Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial. Scientific reports. 2018;8:2784.
- El Brihi J, Horne R, Faasse K. Prescribing Placebos: An Experimental Examination of the Role of Dose, Expectancies, and Adherence in Open-Label Placebo Effects. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2018.
- Locher C, Nascimento AF, Kirsch I, Kossowsky J, Meyer A, Gaab J. Is the rationale more important than deception? A randomized controlled trial of openlabel placebo analgesia. Pain. 2017;158:2320-8
- Trogen B, Caplan A, Klass P. The Ethics of Open-Label Placebos in Pediatrics. Pediatrics. 2017;140, doi: 10.1542/peds.2016-4328.
- Blease C, Colloca L, Kaptchuk TJ. Are open-Label Placebos Ethical? Informed Consent and Ethical Equivocations. Bioethics. 2016;30:407-14
- Miller FG, Wendler D, Swartzman LC. Deception in research on the placebo effect. PLoS medicine. 2005;2:e262.
- Charlesworth JEG, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, et al. Effects of placebos without deception compared with no treatment: a systematic review and meta-analysis. J Evid Based Med. 2017;10:97-107.
- Petkovic G, Charlesworth JE, Kelley J, Miller F, Roberts N, Howick J. Effects of placebos without deception compared with no treatment: protocol for a systematic review and meta-analysis. BMJ open. 2015;5:e009428.
- Ballou S, Kaptchuk TJ, Hirsch W, Nee J, Iturrino J, Hall KT, et al. Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials. 2017;18:234.









