
The Alphavirus Venezuelan equine encephalitis virus (VEEV) was first identified as the causative agent of an equine disease in 1938 and later was found to be a human pathogen. While endemic strains circulating in rodents are generally unable to cause disease in humans, mutations in certain VEEV subtypes can create an epizootic strain, carried by mosquitos, that is capable of infection and spread between horses and humans. Outbreaks can last many years and have been responsible for 100,000s of human cases, and vast numbers of equine deaths.1,2
With VEEV infection largely resulting in symptoms similar to flu, the mortality rate in humans is 1%, but in about 14% of cases, it can lead to a neurological disease with hemorrhages, meningitis, and encephalitis.2,3 The high infectivity of VEEV combined with its ease of manipulation and ability to grow to high titres in host cells has made it attractive as a potential bioweapon, especially as there are no anti-virals against VEEV, and no licensed vaccines approved for general human use. The only human vaccine is based on the TC-83 live attenuated strain and is used for at-risk personnel, including military and researchers, as it provides only limited protection, but with high rates of adverse effects. Undoubtedly, there is a need to develop new treatments to combat future outbreaks.
VEEV capsid protein as a target
In addition to its structural role in binding viral RNA and forming viral particles, the VEEV capsid protein (CP) plays a key role in pathogenicity, where it inhibits the active transport of large proteins into the host cell nucleus, interrupting signal cascades and impeding the host cell’s ability to mount an effective antiviral response.4-6 Active transport of molecules into or out of the nucleus is mediated by host proteins of the importin/exportin superfamily of transporters. CP has been found to bind simultaneously to import and export proteins, specifically the importin α/β1 heterodimer (Impα/β1), and exportin 1.7 Reduced binding of CP to either Impα/β1 or exportin 1 through mutations in CP, or treatment with small molecules inhibiting binding, results in reduced viral pathogenicity, highlighting nucleocytoplasmic trafficking of CP as a potential target for antivirals against VEEV.8
HTS for anti-VEEV agents
Targeting host cell components in the quest for antivirals can lead to unintended side-effects, whereas targeting viral proteins can result in rapid selection for resistance mutations.9-11 In contrast, targeting the host:virus interface limits the scope for viral resistance and minimizes the potential for unwanted side-effects on the host. To this end, we performed high throughput screening (HTS) for small molecules able to inhibit the Impα/β1:CP interaction, with the ultimate aim of identifying new chemical scaffolds with antiviral activity. A novel, nested counterscreen design (Fig. 1) was used, where compounds able to inhibit the Impα/β1:CP interaction were also screened for their ability to inhibit Impα/β1:T-agNLS (another Impα/β1 binding partner) binding or an assay control. This allowed us to identify specific compounds of interest that inhibited Impα/β1:CP binding, but did not inhibit Impα/β1 binding to non-VEEV cargo, or interfere with the assay itself.
From a library of 14,468 compounds, multiple compounds were identified that showed strong activity against the Impα/β1:CP interaction while minimally inhibiting Impα/β1:T-agNLS binding (Fig. 2). Several of these compounds were able to significantly reduce viral replication in infected cells, with EC50s (the concentration of drug required to reduce viral replication by 50%) in the low µM range (Fig. 3), and inhibit the production of infectious TC83-VEEV particles while showing minimal toxicity. Live cell imaging also demonstrated their ability to inhibit CP nuclear import, while not affecting the nuclear import of another Impα/β1 cargo, further underlining the specificity of the hit compounds (Fig. 4).
Conclusions and Future directions
This study proves the principle that selective inhibitors of CP nucleocytoplasmic trafficking can be potent antivirals against VEEV, and represents a platform for future development of safe anti-VEEV compounds with high efficacy and specificity. Given that the CP sequence is highly conserved amongst New World Alphaviruses, it would also be exciting to test our compounds against the related Eastern and Western Equine Encephalitis viruses, as well as wild-type strains of VEEV.
Most importantly, our HTS approach, based on nested counterscreens designed to enable the rapid identification of selective, active inhibitors, represents a platform that can easily be used for various other viruses, and indeed other pathogens. As perturbed nucleocytoplasmic transport is not only related to viral infections but is also implicated in the development of certain cancers and neurodegenerative diseases, HTS could yield valuable drugs across a vast range of diseases. In fact, we have already been successful in identifying potent antivirals against Dengue and Zika viruses, using this nested HTS approach.10-12
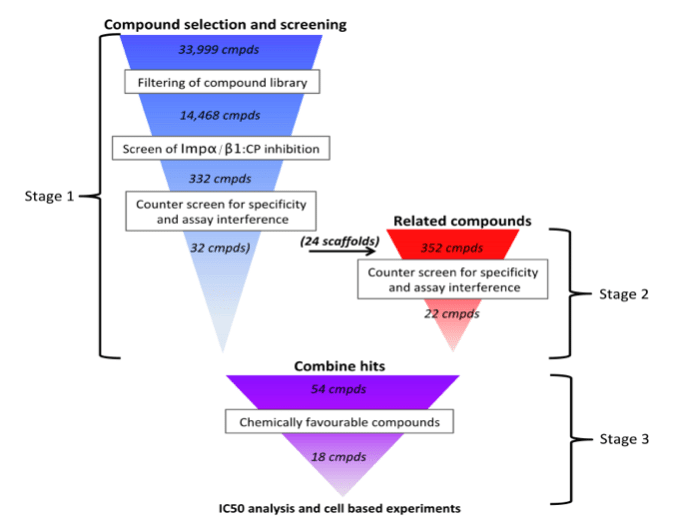
Figure 1. HTS design. Stage 1) The 33,999 compounds in the Open Scaffold library were filtered to select 14,468 compounds amenable to drug development, and screened for their ability to inhibit the Impα/β1:CP binding using AlphaScreen technology, followed by counter-screening to remove those showing significant off-target effects (inhibition of Impα/β1:GFP-T-agNLS) or assay interference (inhibition of His6-GST) in the AlphaScreen system. Stage 2) Compounds structurally similar to selective hits from Stage 1 were tested using the same screen/counter-screen procedure. Stage 3) Compounds were selected for further analysis based on their compatibility with drug-development, and validated in cell-based experiments. (Credit: David R Thomas)
Figure 2. Selectivity of hit compounds. Distribution of the inhibition of signal of the top 332 initially screened compounds in the Impα/β1:CP (left) and Impα/β1:T-agNLS (right) AlphaScreens as indicated. (Credit: David R Thomas)
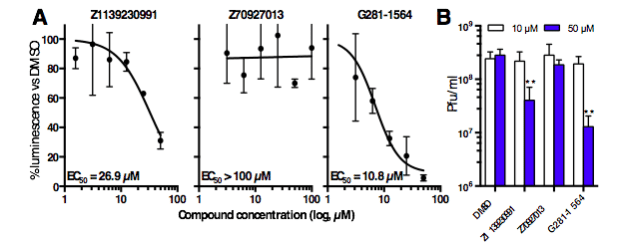
Figure 3. Lead compounds inhibit viral replication and virulence. (A) Cells were treated with DMSO supplemented with increasing concentrations of compounds prior to infection with VEEV expressing the reporter gene luciferase. Luminescence in the continued presence of compounds was expressed as a percentage of that of DMSO-only treated cells. (B) Vero cells were treated with DMSO supplemented with 10 or 50 µM compounds, and were infected with TC83-VEEV. Infectious viral titers were measured by plaque assay. ** p < 0.01 (Credit: David R Thomas)
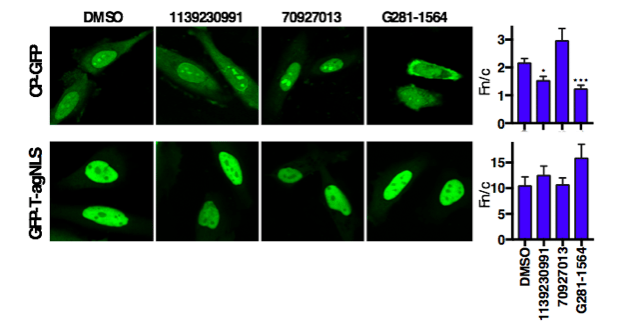
Figure 4. Hit compounds selectively inhibit CP nuclear accumulation. HeLa cells transfected to express CP-GFP or GFP-T-agNLS were treated with 50 µM compound or DMSO as indicated for 20 h prior to imaging. The ratios of nuclear to cytoplasmic fluorescence, Fn/c, of transfected cells were determined and compared to DMSO treated controls. * p < 0.05; *** p < 0.001. (Credit: David R Thomas)
These findings are described in the article entitled Identification of novel antivirals inhibiting recognition of Venezuelan equine encephalitis virus capsid protein by the Importin α/β1 heterodimer through high-throughput screening, recently published in the journal Antiviral Research.
This work was conducted by David R. Thomas, Kylie M. Wagstaff, and David A. Jans from Monash University; Aaron DeBono and Caroline A. Hick from the Monash Institute of Pharmaceutical Sciences; Jonathan Baell from the Monash Institute of Pharmaceutical Sciences and Nanjing Tech University; Sharon Shechter from Shechter Computational Solutions; and Lindsay Lundberg, Chelsea Pinkham, and Kylene Kehn-Hall from George Mason University. This work was funded through a Defense Threat Reduction Agency grant, HDTRA1-15-1-0014, to KKH, DAJ, JB, and KMW. DTRA does not have any role in the design of the study and collection, analysis, and interpretation of data and nor in writing the manuscript.
References:
- Navarro, J. C. et al. Postepizootic persistence of Venezuelan equine encephalitis virus, Venezuela. Emerg Infect Dis 11, 1907-1915, doi:10.3201/eid1112.050533 (2005).
- Weaver, S. C., Ferro, C., Barrera, R., Boshell, J. & Navarro, J. C. Venezuelan equine encephalitis. Annu Rev Entomol 49, 141-174, doi:10.1146/annurev.ento.49.061802.123422 (2004).
- Johnson, K. M. & Martin, D. H. Venezuelan equine encephalitis. Advances in veterinary science and comparative medicine 18, 79-116 (1974).
- Lundberg, L. et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res 100, 662-672, doi:10.1016/j.antiviral.2013.10.004 (2013).
- Garmashova, N. et al. The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J Virol 81, 2472-2484, doi:10.1128/JVI.02073-06 (2007).
- Garmashova, N. et al. Analysis of Venezuelan equine encephalitis virus capsid protein function in the inhibition of cellular transcription. J Virol 81, 13552-13565, doi:10.1128/JVI.01576-07 (2007).
- Atasheva, S., Fish, A., Fornerod, M. & Frolova, E. I. Venezuelan equine Encephalitis virus capsid protein forms a tetrameric complex with CRM1 and importin alpha/beta that obstructs nuclear pore complex function. J Virol 84, 4158-4171, doi:10.1128/JVI.02554-09 (2010).
- Shechter, S. et al. Novel inhibitors targeting Venezuelan equine encephalitis virus capsid protein identified using In Silico Structure-Based-Drug-Design. Sci Rep 7, 17705, doi:10.1038/s41598-017-17672-9 (2017).
- Caly, L., Wagstaff, K. M. & Jans, D. A. Nuclear trafficking of proteins from RNA viruses: potential target for antivirals? Antiviral Res 95, 202-206, doi:10.1016/j.antiviral.2012.06.008 (2012).
- Wagstaff, K. M., Rawlinson, S. M., Hearps, A. C. & Jans, D. A. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen 16, 192-200, doi:10.1177/1087057110390360 (2011).
- Fraser, J. E. et al. A nuclear transport inhibitor that modulates the unfolded protein response and provides in vivo protection against lethal dengue virus infection. J Infect Dis 210, 1780-1791, doi:10.1093/infdis/jiu319 (2014).
- Wang, C-X., Yang, S.N.Y., Smith, K., Forwood, J.K. & Jans, D.A. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochim Biophys Res Commun 493, 1555-1559 (2017).









