
The prediction of cerium aqueous speciation is relevant in many research fields. Indeed, cerium compounds are used for many industrial applications (e.g. in flat-screen TVs, low-energy light bulbs, and floodlights), which may require the control of cerium aqueous chemistry for their synthesis. The aquatic geochemistry of cerium is also of interest (see my previous article on rare earth elements).

Figure courtesy Olivier Pourret
Nowadays, cerium is considered as an emerging contaminant due to its increasing industrial use and its release into the environment. Cerium is also used as a proxy for (paleo)redox conditions due to its tetravalent/trivalent redox transition. Finally, the tetravalent is often presented as a relevant analog of tetravalent actinides (see their neighboring position in the periodic table of the elements).
Figure courtesy Olivier Pourret
Actinide solution chemistry in aquatic systems relevant for nuclear wastes disposal is influenced by hydrolysis and complexation reactions. Actinides can be found in various oxidation states in aqueous solution. They commonly range from trivalent to hexavalent depending on the actinide (i.e., thorium, uranium, neptunium, plutonium). A change in oxidation state has drastic consequences on actinides aqueous chemistry, solubility, and sorption to natural organic and inorganic particles and colloids. It affects their mobility in natural systems.
Only tetravalent thorium exists in aqueous solutions. Tetravalent uranium and hexavalent uranium prevail in reducing and oxidizing conditions, respectively, the stability field of pentavalent uranium (found in moderately reducing conditions) being rather narrow. Tetravalent and pentavalent neptunium are the prevalent oxidation states of neptunium, but hexavalent neptunium can form in oxidizing alkaline solutions. Plutonium can be found in oxidation states ranging from trivalent to hexavalent in environmentally relevant conditions.
For all these actinide elements, the tetravalent oxidation state is relevant in circum-neutral pH values likely to be found in nature. Tetravalent actinides have a strong affinity toward hydrolysis in aqueous solution due to their high electric charge. Such attractions undergo polynucleation or further lead to colloid formation. Tetravalent actinides’ chemical behavior is highly consistent across the series, as commonly observed for f-elements (4f lanthanides and 5f actinides) in the same oxidation state. They are thus often considered as chemical analogs.
Lanthanides are naturally occurring trace elements in the environment. In contrast to its lanthanide neighbors (i.e., lanthanum and praseodymium), which predominate in trivalent oxidation state, trivalent cerium can be oxidized to tetravalent cerium under oxidizing conditions. Preferential uptake from aqueous solution by natural particles of tetravalent cerium compared to trivalent cerium can lead to the development of a so-called cerium anomaly (implicitly, by comparison with the behavior of its lanthanide neighbors. The presence (under oxidizing conditions) or the absence (under more reducing conditions) of a cerium anomaly in natural samples is widely used as a proxy of (paleo)redox conditions.
Since cerium is also used for many applications, it is now considered as an emerging contaminant that could affect ecosystems when released into the environment. Tetravalent cerium is often presented as a relevant analog of tetravalent actinides. As cerium is not radioactive, experimental studies can be more easily conducted with tetravalent cerium than with tetravalent actinides, which could improve our scientific knowledge on tetravalent actinides environmental chemistry. Therefore, prediction of cerium aqueous speciation is of great interest for different research fields.
However, by contrast with tetravalent actinides, there is much less information regarding tetravalent cerium aqueous chemistry. In fact, it is more appropriate to use tetravalent actinides to shed light on tetravalent cerium aqueous chemistry than the opposite. Indeed, selected thermodynamic constant database, used to predict tetravalent cerium hydrolysis and solubility in water, only contains formation constants for CeOH3+(aq) and Ce(OH)22+(aq). Such a database is almost incomplete; indeed, Ce(OH)4(aq) species should be in equilibrium with tetravalent cerium-(hydr)oxides at neutral and/or basic pH. It results in an underestimation of tetravalent cerium solubility.
More recently, new hydrolysis constants of tetravalent cerium were published in the scientific literature to achieve a Pourbaix diagram (i.e. an Eh-pH predominance diagram). According to these results, under ambient conditions (0.2 atm), tetravalent cerium should prevail at a pH greater than 4.5. It contrasts with tetravalent thorium and lanthanides (in general) sorption studies on manganese oxide, which is known to quickly oxidize trivalent cerium to tetravalent cerium. An almost complete and pH-independent uptake of tetravalent thorium by manganese oxide is observed for pH values between 3 and 11. By contrast, although more efficiently removed from the solution by manganese oxide than its lanthanide neighbors, only partial uptake of cerium (initially trivalent) by manganese oxide is observed under ambient air, and it increases from pH 4 to 6-7.
This suggests that, between this range of pH, although tetravalent cerium is associated with the solid phase (precipitated or sorbed on manganese oxide), trivalent cerium prevails in solution, as also observed in previous studies dealing with plutonium uptake by clays in moderately reducing conditions (where the tetravalent/trivalent plutonium redox couple was involved). Therefore, tetravalent cerium hydrolysis constants previously published in the scientific literature appear to be questionable.
In the article we published in Dalton Transactions, current knowledge about tetravalent actinide aqua-ions structure, hydrolysis, solubility, and colloids formation in water in the absence of complexing ligands was synthesized. Moreover, similarities between the structure of tetravalent cerium and tetravalent thorium/uranium/neptunium/plutonium aqua-ions were highlighted due to quantum chemical calculations. Because available hydrolysis constants and solubility data for tetravalent cerium were found to be partly inconsistent with those of tetravalent thorium/uranium/neptunium/plutonium, new estimated thermodynamic constants for tetravalent cerium were proposed and presented in the table below.
Table 1. Hydrolysis constants of trivalent and tetravalent cerium used in our study (see explanation in Marsac et al., 2017).
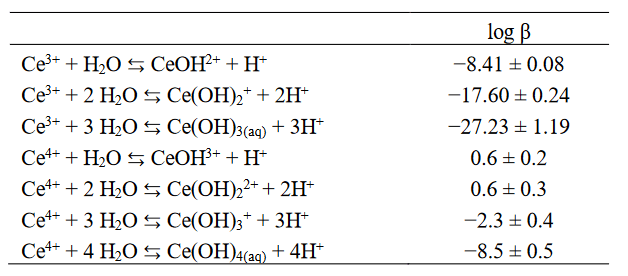
These constants are comparable to those of tetravalent neptunium, plutonium, and cerium. They allow us to predict the speciation as a function of pH of tetravalent cerium compared to tetravalent plutonium (see Figure below).

(A) Comparison between the hydrolysis constants of tetravalent neptunium, plutonium and cerium. The predicted speciation of (B) tetravalent cerium and (C) tetravalent plutonium versus pH are given using the hydrolysis constants selected in our study (see details in Marsac et al., 2017). Figure courtesy Olivier Pourret.
Reference:
- Marsac, R., Real, F., Banik, N.L., Pedrot, M., Pourret, O., Vallet, V., 2017. Aqueous chemistry of Ce(IV): estimations using actinide analogues. Dalton Transactions, 46(39): 13553-13561.
https://pubs.rsc.org/en/content/articlelanding/2017/dt/c7dt02251d/unauth#!divAbstractz









