
Carbon nanotubes (CNTs) may prove to be useful building blocks within the field of nanotechnology. Different nanotubes and molecules might be combined to make useful supramolecular structures for filters, drug storage, nanomechanical devices, etc. However, CNTs have a strong tendency to align in tight, parallel bundles* like a collection of straws stuck in a jar. Although parallel structures are useful for wires or fibers, this aligned clumping makes it challenging to create more open structures as might be used for a filter, for example.
The attraction between CNTs is due to the van der Waals (vdW) forces that are present among any collection of molecules. Van der Waals forces are molecular attractions due to instantaneous induced dipoles. Van der Waals forces are noncovalent (no chemical bonds) and strongest as molecules are close together and “touching.” More surface contact enhances the attraction and so the parallel arrangement of two CNTs gives more vdW interaction than a perpendicular interaction.
In a recent study (1), molecular mechanics was used to model the noncovalent interactions between single-walled carbon nanotubes and molecular linkers. Molecular mechanics calculations are based on classical physics and empirical equations that represent within a molecule the bond lengths, band angles, electrostatic interactions, and so forth. Molecular mechanics is widely used for modeling the three-dimensional structures of molecules. It can also provide information about the interactions between molecules and molecules with carbon surfaces.
This study was interested in the stability of open rather than bundled structures of carbon nanotubes. It had as its goal to create a model of a “nanohashtag” structure that was more stable than any alternate clumps that might form. To construct a nanohashtag, four model CNTs and four molecular linkers were used. The challenge was to find a linker molecule that could hold two carbon nanotubes in a perpendicular arrangement.
Before molecular linkers were introduced to stabilize perpendicular structures of carbon nanotubes, interactions between structures consisting solely of CNTs were compared. The simplest possible structures to compare are two parallel CNTs and two perpendicular CNTs. A model single-walled (5,5) CNT with an internuclei diameter of 0.67 nm and an internuclei length of 4.46 nm (18 rings) was used. Two of these CNTs in a perpendicular orientation are stabilized by −17.7 kilocalories per mole (kcal/mol). However, two of these CNTs in a parallel orientation are stabilized by −83.1 kcal/mol. The stabilization or binding energy is a comparison of the final structure relative to the noninteracting component parts. A parallel bundle of four CNTs is stabilized by -400 kcal/mol (-17.4 eV per single structure) whereas a hashtag structure (#) is stabilized by only -71 kcal/mol (-3.1 eV per single structure).
Dynamic view of CNT capture by molecular linker (using Scigress software for modeling)
To further stabilize the hashtag structure, it was necessary to introduce molecular linkers capable of binding and holding two CNTs in a stable perpendicular orientation. A set of linkers were designed and built around a 1,3,5,7-cyclooctatetraene molecule with two pincers that extend in opposite directions from the central cyclooctatetraene portion. Each pincer consists of a pair of “arms.” These molecular linkers were modified so that the “hand” portions of each pair of “arms” could close together to grab and hold two carbon nanotubes in a perpendicular arrangement. The pincers had to be enlarged to maximize vdW interactions and contact with the “grabbed” CNT (see Figure 1). Additional bridging groups on each side of the pair of pincers were later added to keep the pincers arms from collapsing together.
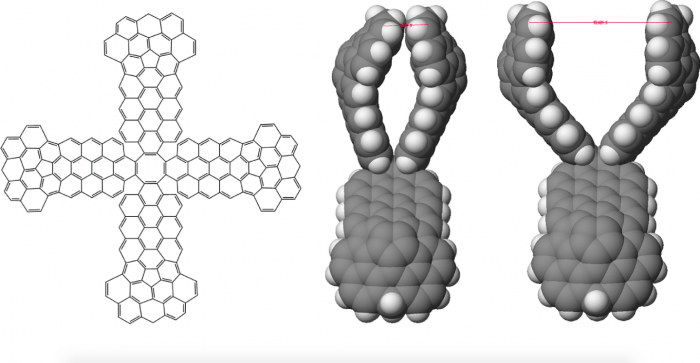
Figure 1. (figure 6 in original article) Line drawing representation of basic structure and space filling representations of open and closed pincer. Not shown are bridging groups added to help hold pincer with its two “arms” in an open position to be ready to grab a carbon nanotube. Reprinted from https://doi.org/10.1016/j.susc.2017.11.005 with permission from Elsevier.
The stable hashtag structural goal was achieved using a molecular linker (C280H96) that utilizes van der Waals interactions to hold two perpendicular oriented CNTs. A nanohashtag structure using C280H96 was calculated to have a stabilization energy of −814 kcal/mol (see Figure 2). This nanohashtag was compared to various random clumps made from the four CNTs and the four linkers, and no random structure including various parallel bundles was as stable as the desired nanohashtag.
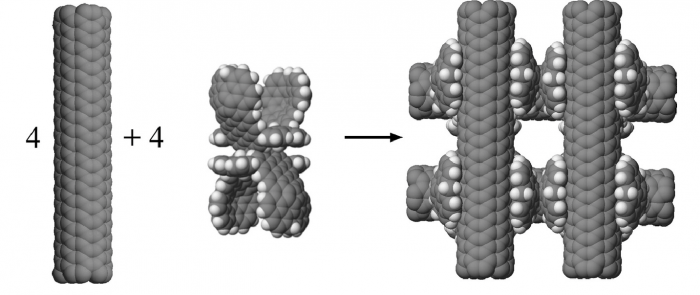
Figure 2. (Graphical Abstract in original article) Four carbon nanotubes and four molecular linkers combine to form a stable nanohashtag structure. Each molecular linker holds two carbon nanotubes in a perpendicular orientation. Reprinted from https://doi.org/10.1016/j.susc.2017.11.005 with permission from Elsevier.
Hydrogen bonding was then added between linker molecules to augment the stability of the hashtag structure. In the hashtag structure with hydrogen bonding, four (5,5) CNTs of length 4.46 nm (18 rings) and four linkers (C276H92N8O8) stabilized the hashtag so that the average binding energy per pincer was -118 kcal/mol. This arrangement gives a stabilization of -944 kcal/mol per nanohashtag relative to the component parts (four CNTs and four linker molecules). For comparison a single C–H bond requires about 104 kcal/mol to break so this level of stabilization is significant.
Imagining and modeling more extensive assemblies consisting of long CNTs with multiple crosslinking molecules may help lead to new two- and three-dimensional structures and applications. Such applications might include sorting and separating different diameter CNTs based on which CNTs could be held by a specifically designed linker. Also, appropriate noncovalent linkers might be used to provide additional means of attachment for other molecules as well. The nanohashtag structures modeled in this work (1) hopefully serve as an illustration of the possibilities for modeling and building ever more complex supramolecular nanostructures.
These findings are described in the article entitled Nanohashtag structures based on carbon nanotubes and molecular linkers (1) recently published in the journal Surface Science. This work was conducted by Thomas R. Rybolt and Connor W. Frye from the University of Tennessee at Chattanooga. Connor W. Frye currently is a graduate student at the University of Minnesota.
*The tendency of CNTs to form parallel bundles extending even to macroscopic conducting ropes or wires and the ability to enhance this aggregation by external electric fields (Teslaphoresis) has been demonstrated in https://pubs.acs.org/doi/abs/10.1021/acsnano.6b02313








