
Hydrogen production has become increasingly attractive as the application of renewable and sustainable energy systems becomes more widespread to resolve global warming. Basically, water electrolysis is one of the most practical and valuable ways to generate hydrogen gas using electrical power. And then, this generated hydrogen reconverted the electrical power in the fuel cell. Therefore, this hydrogen-electricity circulating system is expected to decrease environmental carbon dioxide compared to alternative the conventional system using thermal or atomic power generation.
Recently, Professor Hiroshige Matsumoto and his coauthors from Kyushu University and the Japan Aerospace Exploration Agency (JAXA) designed and developed a new type of water electrolysis cell, which was named the “water absorbing porous electrolyte electrolysis cell.” Compared with conventional polymer electrolyte membrane water electrolysis cells (PEMWE, Fig. 1 (a)), the structure of this cell is so unique and interesting and consists of an inorganic porous electrolyte layer and hydrophobic electrocatalyst layer as shown in Fig. 1 (b). These structural characters of this cell expect to realize a water electrolysis system with operation at operation with higher energy efficiency, lower production (and maintenance) cost, and various practical applications.
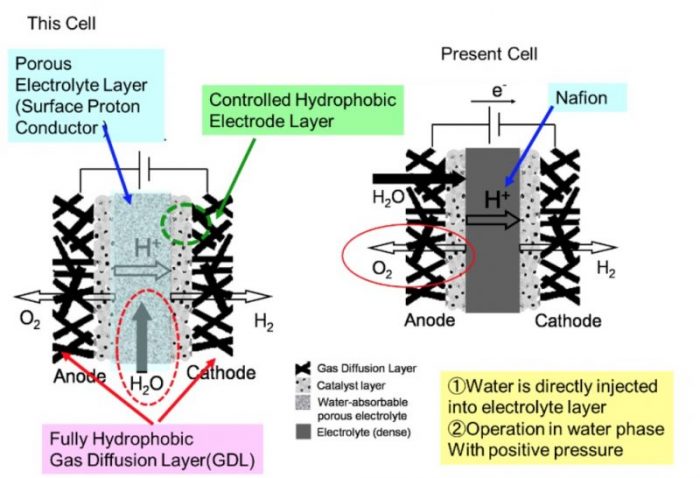
Fig.1 The electrolysis cell structure (a) conventional PEMWE cell and (b) water absorbing porous electrolyte electrolysis cell. Credit: Yuki Terayama
However, in the primary stage of this study, the sufficiently required inorganic porous electrolyte layer and hydrophobic electrocatalyst layer for water absorbing porous electrolyte electrolysis cells had not been developed yet. In many experiments for over 10 years, the suitable electrolyte layer and electrocatalyst layer were successfully prepared. In part of the electrolyte layer, protonated layered titanium oxide nanoparticle treated by crushing planetary bead-milling process showed higher electrical conductivity than 1.0 × 10-2 S cm-1 , a value which is relatively reasonable for a water electrolysis cell.
On the other hand, in part of the electrocatalyst layer, the electrocatalyst/PTFE/PVDF were uniformly mixed and were successfully film-formed on the carbon paper using transfer-method as shown in Fig. 2. That prepared electrocatalyst layer shows working for maintaining under over 1.0 MPa of water pressure and suitable gas permeability of generated hydrogen and oxygen gases during water electrolysis.

Fig.2 Hydrophobic electrocatalyst layer prepared by the transfer method. Credit: Yuki Terayama
In addition, we identified that an electrolysis cell equipped with that electrolyte layer and electrocatalyst layer (Fig. 3) actually operated the first time. The result of the water electrolysis test in Fig. 4 showed a relatively lower I-V curve and a hydrogen evolution rate that obeyed with Faraday’s law. Although further optimization of this cell is required, we are continuing to develop this water absorbing porous electrolyte electrolysis cell to achieve the operation at a practical level and further in the future.
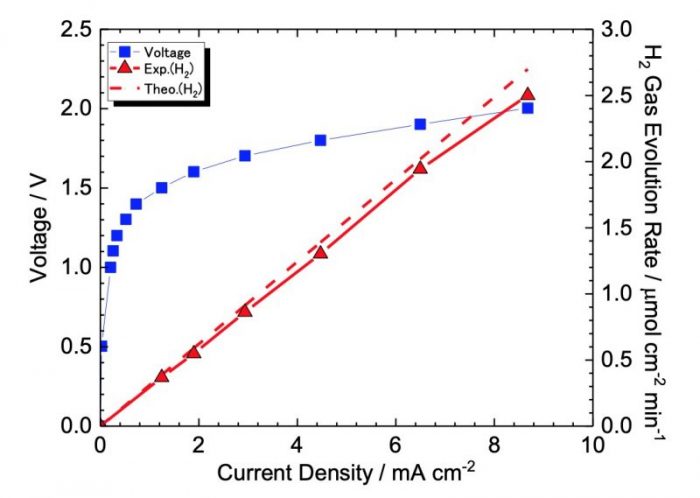
Fig. 4 The result of water electrolysis test using electrolysis cell with hydrophobic electrocatalyst layer and electrolyte layer. Credit: Yuki Terayama
These findings are described in the article entitled Preparation of hydrophobic electrocatalyst layer and inorganic porous electrolyte layer for water absorbing porous electrolyte electrolysis cell, recently published in the International Journal of Hydrogen Energy. This work was conducted by Yuki Terayama, Shoichi Furukawa, Munemitsu Nomura, Takamasa Haji, Masamichi Nishihara, and Hiroshige Matsumoto from Kyushu University, and Omar Mendoza and Yoshitsugu Sone from the Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency (JAXA).








