
The molecular dihydrogen (H2) is considered as a zero-emission fuel, as it produces water vapor when it burns, causing no damage to our environment and can be made renewable. Another positive aspect of its use is that it has the highest energy content among the known fuels. The heat value for H2is 120-142 MJ∙kg-1.[1] However, the present geopolitical situation that strongly influences the supply and cost of fossil fuels, demands that H2 be looked into as an alternative energy source for mobile applications. Though H2 is one of the most abundant elements on earth, it is chemically bound in water and hydrocarbons.
To use H2 as a fuel, first, we need to free molecular H2 from its source, store it in proper storage systems, and transport it to the fuel stations. However, storing H2 causes the major complication in the whole process. In this regard, several materials like nanotubes, graphenes, fullerenes, metal clusters, metal hydrides, clathrates, metal-organic framework (MOF), covalent organic framework (COF), different porous silica, etc.have been studied extensively.In order to take care of all these factors, the US Department of Energy (DOE) implemented some necessary conditions what a material should fulfill so that they can be considered to be effective hydrogen storage media.
These criteria include: (i) high stability and lightweight, (ii) small cost and ease of availability, (iii) capability to achieve large volumetric and gravimetric densities of hydrogen, (iv) favorable adsorption and desorption kinetics, and (v) proper thermodynamic parameters so that the material may be considered to be effective in storing hydrogen for mobile applications. The DOE has also set targets for hydrogen storage materials as 45, 55 and 65 g H2/kg system of gravimetric density and 30, 40 and 50 g H2L-1system of volumetric capacity to be achieved in 2020, 2025 and ultimate, respectively.[2]
Extensive use of the fossil fuel along with the emission of the hazardous greenhouse gases are two of the major threats being experienced by mankind in the modern era. The concentration of carbon dioxide (CO2) in our environment is increasing at an alarming rate. The CO2 concentration in air has been recorded to be 408.71 ppm in July 2018. The atmospheric lifetime for the CO2 is 30-95 years. CO2 is the prime suspect for causing the global warming. The major sinks that can remove the CO2 from the atmosphere include the forests, oceans, and other geological reservoirs. The urbanization is causing a rapid decrease in the forest area. Thus, it is high time to think about chemical ways that can reduce the CO2 concentration in our environment. CO2 provides us with an attractive way to generate C1 building unit to the synthetic chemistry. But the inertness of CO2needs special catalysts and treatments by the chemists.
Metal-organic framework (MOF) is one of the most promising materials that can store H2and can fulfill the DOE target. The MOFs are porous materials, synthesized from metal cations and organic linkers. The metal cations and linkers are connected in a repeating fashion. The coordination number of the metal cations determines the morphology of the MOFs. The porosity of the MOFs can be tuned by changing the organic linkers. Further, the metal sites in the MOFs can activate the inert CO2 molecule and can force it to react with and to get transformed into other precursors.
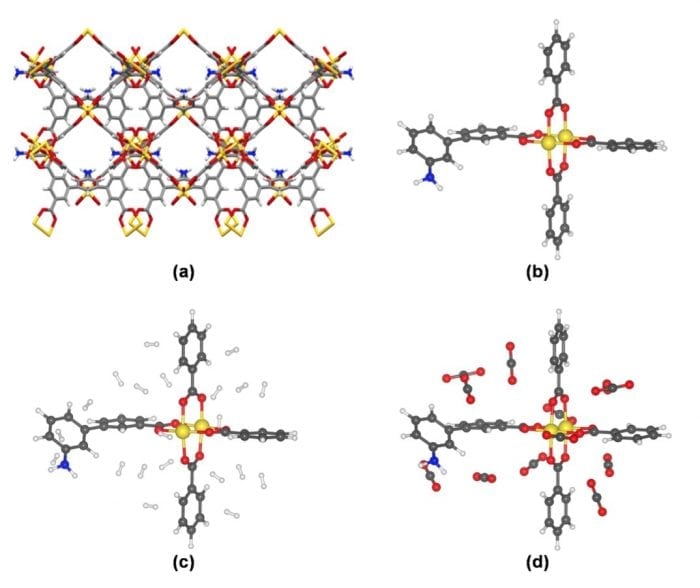
Figure: A depiction of (a) the Cu(II)-MOF in its periodic crystal form; (b) the simplified Cu(II)-MOF; (c) the H2 adsorbed and (d) the CO2 adsorbed in the simplified Cu(II)-MOF. The color code is as follows: yellow for Cu atom, grey for C atom, white for H atom, blue for N atom, red for O atom.
In our recent studies, [1], [2] a Cu(II)-MOF has been synthesized and it is found to adsorb a high amount of H2 and CO2 gases. A combined computational and experimental study has been carried out to study and to understand the adsorption -desorption phenomena through the gas adsorption potential of this MOF.
A solvothermal technique has been used to form the solvent-free highly porous Cu(II)-MOF. The Brunauer-Emmett-Teller (BET) surface area is 1480 m2g-1 with a pore volume of 0.88 cm3g-1. The H2gas adsorption potential of this Cu(II)-MOF is 49 g H2L-1 i.e., 6.6 wt% at 77 K temperature and 62 atm pressure, which is one of the highest recorded H2 storage capacities till date. This high H2storage capacity is close to the target set by DOE. The CO2 gas adsorption capacities in this Cu(II)-MOF at 1 atm pressure are 36.6 wt% at 195 K, 31.3 wt% at 273 K and 17.7 wt% at 298 K. The isosteric heat value (Qst) for the CO2 is 26.72 kJ∙mol-1, which indicates the favorable interactions of the gas molecules with the Cu(II)-MOF. The absorbed CO2 reacts with epoxides and forms corresponding cyclic carbonates. The open metal sites of the MOF activate the CO2gas molecules.
The binding energy per H2gas molecule is -1.6 kcal∙mol-1and per CO2 gas molecule it is -5.0 kcal∙mol-1. These negative values suggest that these gas molecules will remain adsorbed inside the MOF. The non-covalent interactions are major stabilizing factors for the gas absorption processes. Temperature-pressure (T-P) phase diagrams are generated for the relevant gas adsorption-desorption processes. These T-P phase diagrams clearly show two distinct temperature-pressure regions where adsorption or desorption is spontaneous. It is shown that low temperature and high pressure are necessary for favorable adsorption of the gas molecules. Further, a comparative analysis points out that absorption of H2needs much lower working temperature as compared to that of CO2. An ab-initio molecular dynamics study supports this fact. For H2adsorbed Cu(II)-MOF, at 1 atm pressure, the desorption rate at 77 K temperature is higher than that at 4 K temperature. For the CO2 adsorbed system, the CO2 molecules remain adsorbed even at an elevated temperature of 298K implying that the desorption rate is very low.
This Cu(II)-MOF has the potential to be used as an efficient H2 and CO2 storage material which can also sequestrate the CO2 gas molecules.
These findings are described in the article entitled A (T–P) phase diagram for the adsorption/desorption of carbon dioxide and hydrogen in a Cu(II)-MOF, recently published in the journal Polyhedron. This work was conducted by Ranajit Saha from the Indian Institute of Technology Kharagpur, Vivekanand Sharma, Dinesh De, and Parimal K. Bharadwaj from the Indian Institute of Technology Kanpur, and Pratim K. Chattaraj from the Indian Institute of Technology Kharagpur and the Indian Institute of Technology Bombay.
References:
- https://www.world-nuclear.org/information-library/facts-and-figures/heat-values-of-various-fuels.aspx
- https://energy.gov/sites/prod/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf
- V. Sharma, D. De, R. Saha, R. Das, P. K. Chattaraj and P. K. Bharadwaj, “A Cu(II)-MOF Capable of Fixing CO2 From Air and Showing High Capacity H2 and CO2 Adsorption” Chem. Commun. (2017), 53, 13371-13374.
- R. Saha, V. Sharma, D. De, P. K. Bharadwaj and P. K. Chattaraj, “A (T-P) Phase Diagram for the Adsorption/Desorption of Carbon Dioxide and Hydrogen in a Cu(II)-MOF” Polyhedron (2018) 153, 254-260.








