
All soils harbor micro-aggregates. These micro-aggregates composed of smaller building units such as minerals or organic and biotic materials that become more complex with increasing size. In this context, organo-mineral associations can be considered as structural units of soil aggregates and thereby play a fundamental role in the biogeochemical process of soils.
Soil minerals and organic matter (OM) also possess a very high metal-binding capacity, primarily owing to large surface areas and abundant surface reactive adsorption sites. Therefore, organo-mineral associations may significantly change the sequestration of trace elements at the multi-component interfaces, which has a profound impact on the biogeochemical cycling of trace metals in natural environments.
Figure 1. Understanding Cu behavior in model organo-mineral associations is essential for predicting its mobility and fate in soils. Republished with permission from Elsevier from https://doi.org/10.1016/j.chemgeo.2018.09.026.
Huihui Du, associate professor of Environmental Science from Hunan Agricultural University and collaborators focused on this question and identified how copper behaves in a complex Al-OM assemble in a recent study titled “Sorption of Cu(II) by Al hydroxide organo–mineral coprecipitates: Microcalorimetry and NanoSIMS observations,” published in the journal Chemical Geology. We aimed to provide a better understanding of Cu behavior in Al-rich soils and sediments and to present the major unknowns in current organo-mineral association research.
In a well-controlled system, we replicated Al-OM coprecipitate formation in soils and sediments by synthesizing composites using Sigma-Aldrich humic acid during coprecipitation with Al hydroxide. We observed a rapid precipitation process to form an emulsion liquid. Using a high-resolution scanning electron microscope, we find amorphous aggregated Al-OM nanoparticles. The X-ray diffractometer also confirmed the non-crystalline structure. The close association of Al-OM may result from the electrostatic attraction and surface complexation between Al-OH and COOH.
When Cu enters the Al-OM assemble suspension, adsorption occurs rapidly within several minutes. Cu adsorption is more favored at high pH values. The Al-OM assembles sorb more Cu than pure Al counterpart, possibly attributed to the additional binding sites that present in the OM, such as carboxyl groups.
One particular interesting to know is how Cu distributes in the complex Al-OM assembles, i.e., whether Cu likes Al phases or the OM phases. Using Nano secondary ion mass spectrometry, we drew an elemental distribution map of Cu, Al, and C. Interestingly, Cu likes the OM fraction at low pH while Cu prefers the Al phases at high pH. Another important aspect is the order of the adsorption sequence. Using isothermal titration calorimetry, we found that Cu first adsorbs to the OM fraction, then adsorbs to the Al fraction.
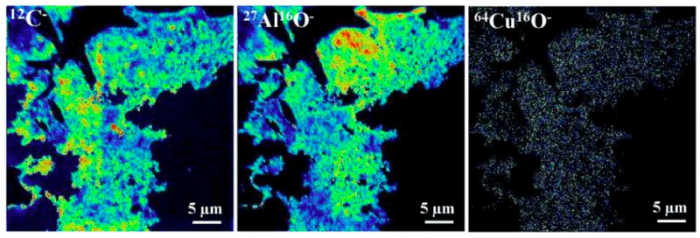
Figure 2. Elemental distribution of Cu within the Al-OM assemble obtained by NanoSIMS. Republished with permission from Elsevier from https://doi.org/10.1016/j.chemgeo.2018.09.026.
This study increases the understanding of the Al–OM interactions and is beneficial for the construction of future thermodynamic models, which can be used to help interpret Cu geochemical signals in Al/organic-rich geologic environments. Finally, we hope to inspire a novel cohort of soil scientists so they might focus their research on improving our understanding of the role of organo-mineral associations within the system of aggregates and help to develop a unified and quantitative concept of aggregation processes in soils.
These findings are described in the article entitled Sorption of Cu(II) by Al hydroxide organo–mineral coprecipitates: Microcalorimetry and NanoSIMS observations, recently published in the journal Chemical Geology. This work was conducted by a team including Huihui Du, Boqing Tie, and Ming Lei et al., from Hunan Agricultural University, and Qiaoyun Huang from Huazhong Agricultural University.
Reference:
- Du H*, Huang Q, Zhou M, Tie B, Lei M, Wei X, Liu X. (2018) Sorption of Cu(II) by Al hydroxide organo–mineral coprecipitates: Microcalorimetry and NanoSIMS observations. Chemical Geology, 499, 165-171. doi: https://linkinghub.elsevier.com/retrieve/pii/S000925411830473X.









