
Photocatalysis is an acceleration of a photoreaction in the presence of light by a photocatalyst. As the photocatalyst is exposed to the light (i.e UV light), strong oxidizing radicals will be generated. These commonly-known radicals are hydroxyl (OH•) and superoxide (O2•). It has been well reported that these radicals are responsible for degrading recalcitrant organic compounds into carbon dioxide and water.
There are plenty of photocatalysts that have been studied, and one of the most iconic examples is titanium dioxide (TiO2). Figure 1 shows the mechanism of TiO2 as a photocatalyst. In the beginning, TiO2 is irradiated by light energy and then causes the electron from the valence band to jump into the conduction band. This creates a positive hole (h+) and a negative electron (e–) in the valence and conduction band, respectively. Then, the electron and holes generate oxidising agents to degrade organic pollutants.
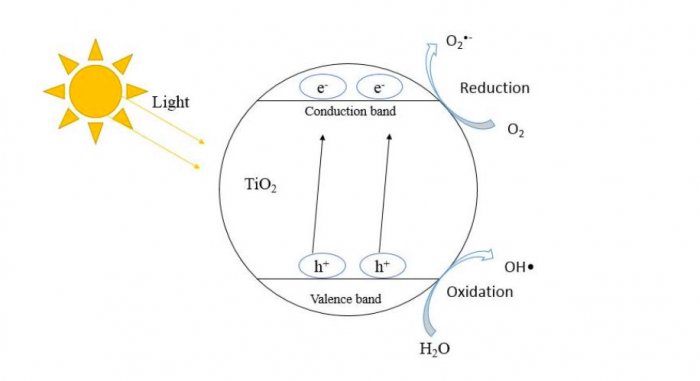
Figure 1: Mechanism of TiO2 during photo-catalysis (Credit: JC Juan)
Although TiO2 manages to photodegrade organic pollutants, it is only effective under UV light, which only makes up 5% of the solar light spectrum. Therefore, many efforts have been made to increase the efficiency of TiO2 into visible light by metal doping such as iron, magnesium, vanadium, etc. In other words, many researchers are adding back an appropriate amount of dopant into TiO2. Nevertheless, there is a lack of studies on natural minerals comprised of TiO2, such as Ilmenite, which already contain the dopant such as iron as a potential photocatalyst.
Ilmenite is a natural mineral, which is also known as Manaccanite, that contains titanium-iron oxide mineral with the idealized formula FeTiO3. The physical attributes and structure of Ilmenite are displayed in Fig. 2. In general, TiO2 is extracted from ilmenite because there is a vast deposit of this mineral in every continent on Earth. Since Ilmenite is comprised of TiO2, could this mineral possess photocatalytic activity?
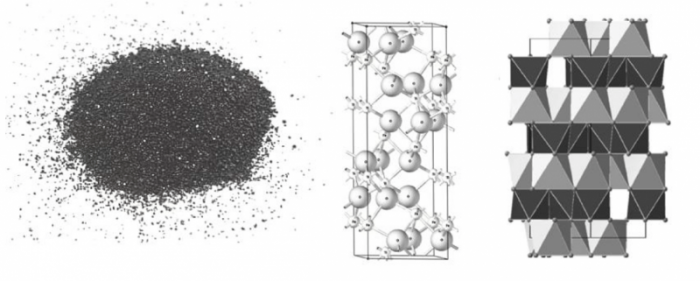
Figure 2: Typical Ilmenite powder and structure of Ilmenite (Credit: JC Juan)
In theory, Ilmenite should have photocatalytic properties thanks to the existence of TiO2. However, the photocatalytic properties of Ilmenite could be lower than that of TiO2 due to the presence of iron ions and other foreign materials in its structure. On the other hand, iron ions could also create easy passage for the excited electron to reach conduction bands prior to the formation of OH•; thus, it capable of functioning in visible light range. In 2011, a study was conducted to evaluate the effectiveness of Ilmenite for the photo degradation of phenol. This study did not reveal the full picture of Ilmenite as a photocatalyst because several factors were not taken into account, such as temperature, pH, etc.
Recently, Ilmenite demonstrated a high photocatalytic effectiveness under solar light irradiation which was capable of fully photodegrade reactive blue 5 (an azo dye) in 2 hours. This study showed that the full capacity of ilmenite was revealed under acidic conditions owing to the nature of Ilmenite’s surface charge. Ilmenite had a negative charge surface in neutral surrounding (pH 7) and the surface charge shifted to positive in range of pH 1 – 4, depending on the source of origin. For this reason, the positive charge surface was easy to attract negatively charged azo dye to the surface of ilmenite as the photo-degradation happened on the surface of the catalyst.
In another study, the Ilemnite was pre-treated with hydrochloric acid in order to tune the iron content. This is because high amounts of iron in the structure would retard the photocatalysis because the excess iron serves as a recombination center for excited electrons through quantum tunnelling. Hence, fast recombination will suppress the formation of these radicals.
The pre-treated Ilmenite possesses high photocatalytic activity due to various synergistic effects such as low band gap (2.84 eV), high surface area (37 m2/g), and low recombination rate. The pre-treated Ilmenite with appropriate amounts of iron (1.62 at.%) managed to photodegrade the RB5 dye completely within 30 minutes at pH 3 under solar light irradiation as compared to raw Ilmenite, which only achieved 40% photo degradation under the same conditions. These findings could be the key to revolutionize photocatalysts by using Ilmenite, which is a natural mineral.
These findings are described in the article entitled The relationship between iron and Ilmenite for photocatalyst degradation, recently published in the journal Advanced Powder Technology. This work was conducted by Ru Bin Lee, Kian Mun Lee, and Chin Wei Lai from the University of Malaya, Guan-Ting Pan and Thomas C.K. Yang from the National Taipei University of Technology, and Joon Ching Juan from the University of Malaya and the Monash University Sunway Campus. These findings are also described in the article entitled Ilmenite: Properties and photodegradation kinetic on reactive black 5 dye, recently published in the journal Chinese Chemical Letters. This work was conducted by Ru-Bin Lee, Joon-Ching Juan, Chin-Wei Lai, and Kian-Mun Lee from the University of Malaya.









