
Biomolecules adsorption at metal surfaces often creates a 2D ordering of the adlayers. The adsorption process can induce in some rare cases the reconstruction of the metal substrate. This phenomenon is less commonly observed, unless upon annealing of the molecule/surface system. In this study, we reported on the drastic room temperature reconstruction of the Au surface driven by the adsorption of tripeptides, observed earlier (1). STM images showed that the surface reconstruction, taking place without annealing the system, was dynamic and evolving over time. The surface reconstruction was seen to be initiated at kinks and step edges but also remarkably within defect-free terraces.
In order to investigate this phenomenon, we chose the adsorption of GLY-PRO-GLU tri-peptide on Au(110) surface within the frame of all-atom classical mechanics simulations and Density Functional Theory (See Figure 1). It was shown that the tri-peptide adsorption reorganizes and restructures the Au(110) surface. A mechanism for this drastic surface restructuration was proposed for both the neutral and zwitterionic form of the peptide at room temperature in Ultra High Vacuum.
It was observed that several residues were involved in the displacement of Au atoms, and in particular, the glutamic acid residue, triggering a double proton transfer and the formation of a zwitter ionic state of the tripeptide, was found to be responsible for the triggering of the surface reconstruction.
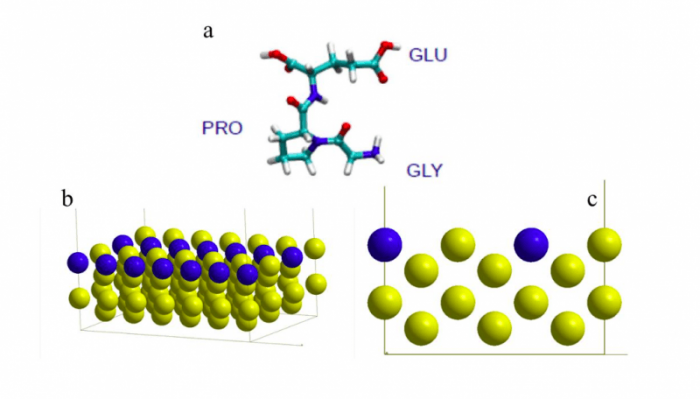
Figure 1. a. Schematic representation of the GLY-PRO-GLU peptide, showing the 3 amino acid residues. (turquoise = carbon, red = oxygen, blue = nitrogen, white = hydrogen), b and c. slab model of the Au(110) surface showing the missing row in blue. Figure taken with permission from (2).
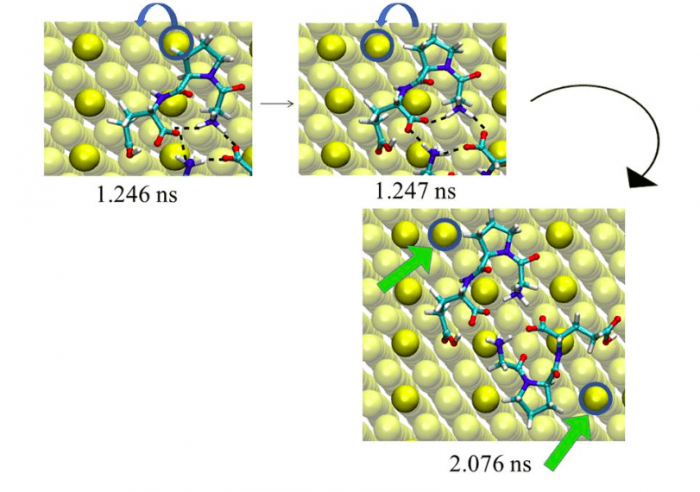
Figure 2. Snapshot of the Au atom displacement on MRR Au(110) initiated by the PRO residue of the zwitter ionic GLY-PRO-GLU tripeptide at t = 2.07 ns. The green arrows show the displaced surface Au atoms. (Au = yellow, turquoise = carbon, red = oxygen, blue = nitrogen, white = hydrogen). Figure taken with permission from (2).
In particular, we showed that that the zwitter ionic peptide tends to form dimers via intermolecular H-bonds. In our MD simulation, the planar dimer displaces a first Au atom after 1.24 ns, with the help of the PRO residue. The second peptide displaces another Au atom after 2.07 ns (See Figure 2). The geometry of the adsorption complex shows mirror symmetry. The peptide dimerization enhances the displacement of the Au surface atoms and thus the surface reconstruction. The described mechanism was observed in several different simulations.
With the information obtained from the MD simulations, we were able to propose a complete adsorption process including the displacement of surface gold atoms. In a first step the tripeptide adsorbs on the surface and the GLU residue undergoes a rotation (See Figure 3a). This configuration enables in a second step a proton to shift from the GLU terminal COOH group to the NH2 end group mediated by the GLU sidechain group (See Figure 3b). This double proton transfer promotes finally the displacement of the Au atom situated next to the tail COO– group of the tripeptide (See Figure 3c).
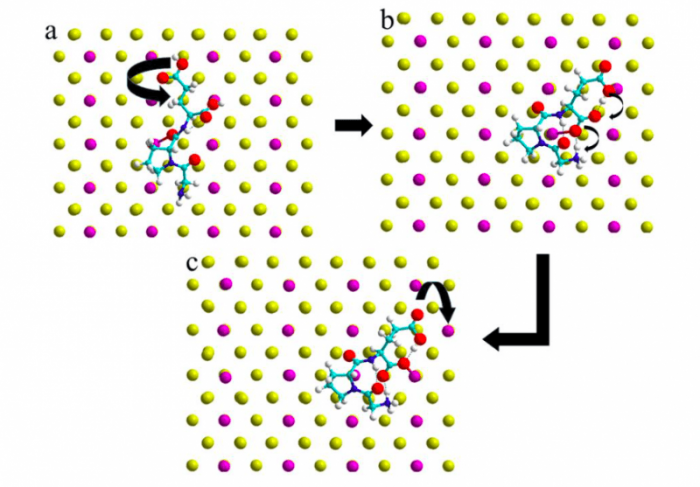
Figure 3. Selected Au hopping mechanism induced by IGF adsorption based on the DFT energetics. Figure taken with permission from (2).
After the MD trajectories were studies we were able to calculate the adsorption energies of the peptide on the Au surface. The neutral peptide adsorbs to the surface (Fig. 3a) with a binding energy of -1.85 eV. The rotation of the GLU residue with the spontaneous double proton shift (Fig. 3b) stabilizes the adsorption by further 0.71 eV. The peptide adsorption liberates surface tension and allows an Au atom to jump from one position to another on the Au(110) surface. This last step occurs almost without additional energy cost (0.04 eV), leading to a final adsorption configuration (Fig. 3c) which has a binding energy of -2.60 eV. This almost barrier less surface reaction is thus easily possible at room temperature, as observed in our simulation and in the experiment9, 25.
Video 1: MD simulation fragment showing the Au atom hopping stimulated by an IGF tripeptide molecule adsorbed on a Au(110) surface.
In conclusion, we showed that IGF tripeptide molecules are adsorbed intact on a Au(110) surface. The molecules are present mainly as zwitterionic chemical forms, with a small fraction of anionic molecules. Based on experimental RAIRS and XPS hypothesis, the adsorption model via both ends of the molecules (NH3+ and COO–) was confirmed as the most stable geometry by DFT calculations. The molecule/surface interaction has been calculated to be 2.5 times higher than the one usually observed for a simple amino acid adsorption in the same chemical form on Au(111) within the same conditions. In fact, IGF molecule adsorption energy is so high that its interaction with a gold atom is stronger than the cohesion energy between the surface gold atoms The IGF-gold atom clusters are thus very mobile on the surface and these reconstructions are taking place at room temperature, without need for a further annealing, at step edges and inside defect-free terraces and they are dynamic over time as shown by successive recorded STM images.
These findings are described in the article entitled Unravelling the GLY-PRO-GLU Tripeptide Induced Reconstruction of the Au(110) Surface at the Molecular Scale, recently published in the journal Surface Science. This work was conducted by Isidro Lorenzo Geada and Marialore Sulpizi from the Johannes Gutenberg University Mainz, Ivan Petit from UPMC Univ Paris, and Frederik Tielens from the UPMC Univ Paris and Vrije Universiteit Brussel.
References
- V. Humblot*, A. Naitabdi, A. Vallée, F. Tielens, C.-M. Pradier. Drastic Au(111) Surface Reconstruction upon IGF Tripeptide Adsorption J. Am. Chem. Soc. 134, 6579 (2012).
- I. Lorenzo Geada, I. Petit, M. Sulpizi and F. Tielens*, Unravelling the GLY-PRO-GLU Tripeptide Induced Reconstruction of the Au(110) Surface at the Molecular Scale, Surf.Sci. 677 (2018) 271.
Corresponding authors:
F. Tielens: [email protected]
M. Sulpizi: [email protected]








