
Over 30 million Americans (roughly 10% of the population) have type II diabetes, a disease characterized by insulin resistance which is ca1used primarily by obesity resulting from overnutrition. However, previous research shows that not all dietary excess is created equally. For example, excess glucose, fructose, and fat all have unique effects in the body.
In an article published in PLOS One, researchers from the Schilling lab at the Buck Institute show that excess dietary fat and sugar, despite a common metabolic intermediate, induce unique molecular changes in the liver that may reinforce metabolism of themselves by inhibiting the metabolism of the other. These findings in mice have implications for the treatment of type II diabetes in humans.
“We know that everything we eat impacts our weight and how we feel, but not all calories are created equally,” said Jesse Meyer, Ph.D., lead scientist on the study. “The fact that both fat and sugar may reinforce the metabolism of themselves by inhibiting metabolism of the other opens a new horizon of research that may improve our ability to manage type II diabetes in humans.”
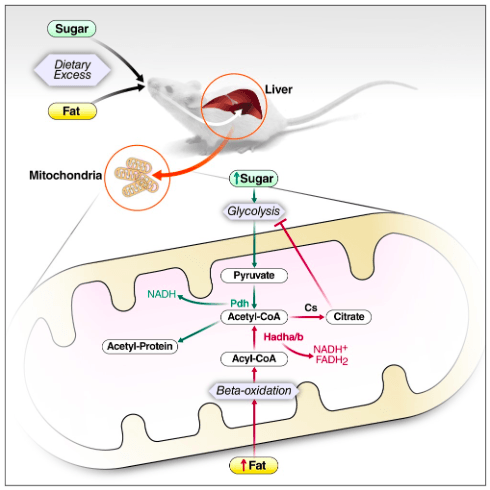
Image republished with permission from PLOS One from doi.org/10.1371/journal.pone.0208973
Dr. Meyer and colleagues used modern technology to perform unbiased measurements of multiple classes of molecules in the mouse liver. Assembly and interpretation of these multi-omic datasets is a daunting task that requires expert knowledge of metabolism. Dr. Meyer observed that molecules downstream of Acetyl-CoA, a metabolic intermediate common to sugar and fat metabolism, were altered differently depending on the source of excess calories.
Researchers also found that excess dietary fat resulted in an accumulation of citrate, a metabolite known to inhibit glycolysis or sugar metabolism. In contrast, excess dietary sugar resulted in an accumulation of a specific protein modification studied for many years by the Verdin and Schilling labs: lysine acetylation.
“Increased modification of mitochondrial proteins by acetylation was famously reported to inhibit fat metabolism in 2010 by Dr. Eric Verdin’s group,” said Jesse Meyer. “Our study is the first we’re aware of in mammals to report that excess dietary sugar (either glucose or fructose) can induce this protein modification.”
This study also looked at the role of another less-studied protein modification in parallel: protein succinylation. Even while they discovered that a high-fat diet decreased global protein succinylation, the function of this change is still unclear.
“This was a perfect project to marry proteomics, metabolomics, and the study of protein modifications to collect unbiased data at the system level,” said Dr. Meyer. “Using this combination of technologies together enables discoveries that would have otherwise been impossible to detect.”
These findings are described in the article entitled Temporal dynamics of liver mitochondrial protein acetylation and succinylation and metabolites due to high fat diet and/or excess glucose or fructose, recently published in the journal PLoS One. This work was conducted by Jesse G. Meyer from the University of Wisconsin-Madison, Samir Softic and C. Ronald Kahn from Harvard Medical School, Nathan Basisty, Eric Verdin, and Birgit Schilling from the Buck Institute for Research on Aging, Matthew J. Rardin and Bradford W. Gibson from Amgen, and Olga Ilkayeva and Christopher B. Newgard from Duke University Medical Center.
The work was funded by NIH grants R24DK085610, T32AG000266, K12HD000850, R01DK033201, P30DK036836.
Citation:
Meyer JG, Softic S, Basisty N, Rardin MJ, Verdin E, Gibson BW, et al. (2018) Temporal dynamics of liver mitochondrial protein acetylation and succinylation and metabolites due to high fat diet and/or excess glucose or fructose. PLoS ONE 13(12): e0208973. https://doi.org/10.1371/journal.pone.0208973
DOI: 10.1371/journal.pone.0208973









