
Mucosa is widely distributed among the human tracts, including nasal cavity, oral cavity, airways, eyes, gastrointestinal (GI) tract and genital tract. For more acceptable and mild administration, mucosa has been a promising alternative to invasive injection.
Unfortunately, despite the excellence of mucosal delivery, limited bioavailability compared with injection still restricts its application and development. Obstacles include the environment with varying pH and numerous enzymes. For instance, robust digestive enzymes in the GI tract severely challenges the stability of macromolecular drugs. Moreover, some drugs with poor solubility cannot achieve good absorption.
To overcome these obstacles, nanotechnology had been widely investigated in this field. By encapsulating drugs into nanocarriers, nanotechnology can protect drugs from degradation and modulate their release. However, due to the unique physiological features of mucosa, the mucus layer becomes a penetration barrier for nanocarriers. Mucus is an adhesive and viscoelastic gel, overlying the epithelium.
In general, there are three major factors influencing the mucus penetration ability of nanoparticles (NPs). Firstly, mucus has a mesh-like structure. Human cervicovaginal mucus has an average mesh spacing (pores) of about 340 nm. Thus, nanocarriers with large size cannot rapidly penetrate through the mucus layer. Secondly, mucins, the key component of mucus, are abundant of negatively charged groups. Thus, positively charged particles are more likely to be trapped in mucus due to the electrostatic interaction. Thirdly, hydrophobic particles are also trapped by hydrophobic interaction with hydrophobic segments on mucins. To sum up, mucus-penetrating particles (MPPs) require three basic criteria: (1) small size, (2) negative or nearly neutral charge, and (3) hydrophilic surface.
During past decades, mucus-penetration has been achieved by two basic strategies. One was the application of mucolytic agents, which decrease the viscosity of mucus by chemical reaction. The other was the poly(ethylene glycol) (PEG) coating for NPs. The PEG shell could avoid the hydrophobic interaction between mucus and NP core when the molecular weight (Mw) and density of PEG were optimal.
After penetrating the mucus layer, nanoparticles have access to the epithelium (Scheme 1). Nanoparticles also need to overcome this absorption barrier to deliver drugs into blood circulation and take therapeutic effect. Unfortunately, surface properties of MPPs are exactly opposite to those of NPs with high membrane-affinity. Positive charge and a lipophilic surface can significantly enhance the membrane-affinity and cellular uptake.
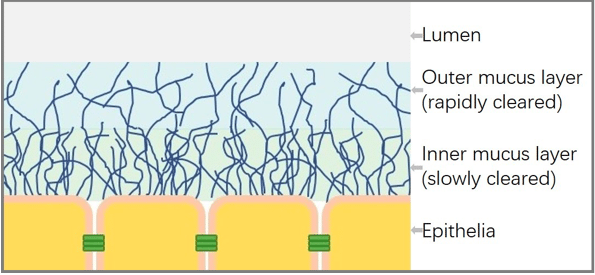
Scheme 1. Summary schematic illustrating the mucus and epithelia (Republished with permission from the journal)
To simultaneously overcome the mucus and epithelium barrier, researchers further modulated the surface properties of NPs. Since the hydrophobic interaction with mucin greatly affected the mucus-permeability, our group regulated the surface hydrophobicity by conjugating hydrophobic segments on the hydrophilic coating polymers. The type and grafting degree of hydrophobic groups were screened to achieve the optimal formulation, which could significantly enhance the mucus-permeability and cellular uptake of hydrophobic core.
Apart from the hydrophobic interaction, the electrostatic interaction was also a key factor. Inspired by the rapid mucus penetration of virus, researchers fabricated a surface with a positive and negative charge of equal amount, which mimicked the feature of proteins on a virus. To further elevate the membrane affinity, researchers developed surface charge changing nanoparticles, which remained negative charge during penetrating mucus layer, while changed to positive charge when reaching the epithelium. Based on this hypothesis, our group has developed a core-shell NP. The negatively charged shell could gradually dissociated during mucus-penetration and the positively charged core would expose in time, which increased the affinity with epithelium.
To sum up, the surface properties had great influence on the efficiency of mucosal drug delivery. Different from traditional MPPs, NPs with the ability to simultaneously overcome the mucus and epithelium barrier have attracted increasing attention. Considering the final target destination, these NPs may facilitate the delivery of drugs into blood circulation and enhance the therapeutic efficacy.
These findings are described in the article entitled Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties, recently published in the journal Advanced Drug Delivery Reviews. This work was conducted by Lei Wu, Wei Shan, Zhirong Zhang, and Yuan Huang from West China School of Pharmacy, Sichuan University.








