The concept of stimulating the immune response of the body against an antigen is the foundation for vaccination. A vaccine adjuvant is an important component of vaccines that primes the immune system to recognize the antigen. The demand for new types of vaccine adjuvants is expected to grow enormously by 2025 due to advances in treatment options for cancer and other conditions. Therefore, it is urgent to develop effective, economically viable, and safe materials that can act as vaccine adjuvants.
Our research is focused on providing comprehensive mechanistic insights into the immunological/inflammatory activity of a variety of surface-charged decorated nanomaterials composed of cellulose nanocrystals (abbreviated as CNCs), using inflammatory cells grown in a Petri dish, also called in vitro. This stepping-stone of basic research contributes to the understanding of the mechanisms of immunomodulatory activity in these nanomaterials and reinforces their potential applicability as vaccine adjuvants. Overall, the knowledge generated through our research can form a solid foundation for evaluating the safety and biological interactions of newly surface-charged decorated CNCs for application as vaccine adjuvants.
Cellulose-based nanomaterials, such as CNCs, have emerged as a new class of renewable nanosized materials for potential biomedical applications from drug and gene delivery systems to tissue scaffolding, among others. These tiny rod-like crystals (3-20 nm wide, 50-3000 nm long) are extracted from the most abundant polymer on earth, native cellulose (trees, plants, algae, tunicates, bacteria), and converted to a powder (Figure 1a) or suspension that can be used for a number of applications. The chemical structure of CNCs consists of linear polymer chains of ß-1−4 linked D-glucose (Figure 1b) arranged in a highly crystalline cellulose I structure. CNCs are attractive nanomaterials because they are renewable, biocompatible, sustainable, high strength, and have a large surface area and low health and environmental risks. Moreover, the presence of numerous exposed surface hydroxyl (-OH) groups allows for easy surface chemical modifications in the design of functional CNC materials with unique properties.
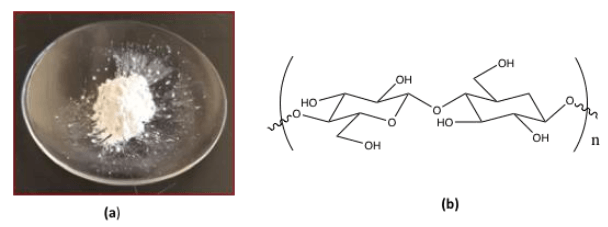
Fig. 1: (a) CNC powder, courtesy Sunasee lab. (b) chemical structure of CNC. Credit: Dr. Rajesh Sunasee
In our study, we took advantage of these reactive -OH groups to decorate the surface of CNCs with different molecules or polymers to generate functional CNCs with varying compositions, sizes, and surface charges. The addition of different decorations on the surface of CNCs can generate compounds with distinct physical and chemical properties and, as a consequence, creates CNC derivatives with a wide range of biological activities. This emerging interest in CNCs for biomedical purposes and their flexibility to be modified chemically led us to synthesize a series of CNCs containing surface-positive charges initially to be used as a gene nanocarrier. However, we quickly realized that their distinct biological activities could be used to develop new biocompatible cellulose-based vaccine nano-adjuvants.
Adjuvants, in the context of vaccines, are defined as components capable of enhancing and/or shaping antigen-specific immune responses. Vaccine adjuvants activate complex signaling networks in a variety of immune cell populations,which are necessary to develop antigen-specific adaptive immunity (1,2). There are only two adjuvants — alum and AS04 — that are used in the majority of the commercially available vaccines in the United States, according to the NIAID website. In contrast, there are more than 2 dozen available vaccines against various types of bacteria and viruses to prevent a variety of infectious diseases. For instance, ligands of toll-like receptors (TLRs) can act as an adjuvant in conjunction with the vaccine to increase the efficacy and response to the immunization with a particular antigen (3). The engagement of TLRs can lead to the subsequent activation of the NOD-like receptor, pyrin domain-containing 3 (NLRP3) inflammasome (NLRP3) and consequent interleukin 1β (IL-1β) secretion.
Although some controversies exist in the current literature, particulate adjuvants, including alum, are also reported to activate the NLRP3 inflammasome/IL-1β pathway (4). Aluminum salts have been employed as adjuvants in human vaccines for many decades. They consist of crystalline nanoparticles that aggregate to form a heterogeneous dispersion of particles of several microns. They are highly charged and conducive to the adsorption of antigens or immunomodulatory molecules. Other particulate molecules such as ISCOMATRIX (IMX) can also act as an adjuvant by evoking this inflammatory pathway. IMX is comprised of phospholipid, saponin, and cholesterol that form “cage-like” structures ∼40 nm in diameter, which potently activates the NLRP3 inflammasome in antigen-presenting cells (APCs), leading to IL-1β and IL-18 production (4). A recent review indicates that new advancements in vaccine design include more in-depth knowledge regarding innate pattern recognition receptors (PRRs) and other molecules responsible for inducing immune responses, as well as developments in the design, synthesis, and characterization of engineered nanomaterials (5).
Initially, we tested these newly-created CNC derivatives for cytotoxicity, i.e. if they can kill cells grown in the Petri dish. We found that, only at very high concentrations, some of these nanomaterials can cause very minimal cell death. However, we found that these CNC derivatives can also evoke an immune response in vitro, depending on the type (positive or negative) and amount of the surface charge. The immunostimulatory activity of positively charged CNC derivatives was first described in our previous work, where we found that cationic CNC derivatives induced the secretion of the inflammatory cytokine, IL-1β, in mouse and human inflammatory cells cultured in a Petri dish (6).
These findings are not a total surprise because there are reports showing that some crystalline nanoparticles, especially those that are positively charged, can evoke an inflammatory/immunological response in vitro by inducing the secretion of the inflammatory cytokines, which can be considered inflammatory mediators. More recently, we observed that negatively charged CNCs also can elicit an immune response similar to the in vitro model. This initial finding prompts us to go further and investigate the mechanism by which these engineered nanomaterials exert their immunomodulatory properties to further explore their potential biomedical application as vaccine adjuvants.
Continuing our studies, we discovered that, depending on the types and charges of surface decorations on the CNCs, this will result in different types of nanomaterials and, hence, will elicit different intensity of immune response by distinct mechanisms. In fact, we described recently that in either positively charged or negatively charged CNCs, although the immune response can be similar, the cellular mechanisms are quite different. In summary, we found that both caused secretion of inflammatory cytokine, however, the negatively charged CNCs induced greater formation of acidic vesicular organelles, perhaps indicating greater engulfment, and the positively charged CNCs mainly affected the function of mitochondria by decreasing the intracellular production of energy (Figure 2) (7). The differences in the biological responses may be related to the surface charges of CNCs and how they interact with the cells and their molecules and organelles. At the microscopic level, these positively or negatively charged CNCs appear to interact with cells differently, causing different types of nanomaterial-cell aggregates (8).
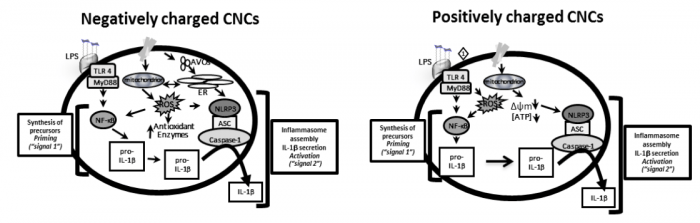
Fig. 2: Proposed mechanisms by which negatively and positively charged CNCs exert their immune responses. The negatively charged CNCs induce in greater extension the formation of acidic vesicles organelles (AVOs) which can cause endoplasmic reticulum stress response and mitochondrial-ROS, which could contribute at least in part to the increases in IL-1β secretion. The positively charged CNCs mainly affect mitochondrial function by disturbing membrane polarization and decreasing intracellular ATP (cellular energy), which could contribute to greater effect on the NLRP3 inflammasome activation and IL-1β secretion. Image modified and published with permission from Elsevier from https://doi.org/10.1016/j.tiv.2018.12.009
Recent advances on the understanding of cellular responses to adjuvants and antigens allow for more rational vaccine design, improving safety and efficacy. One key aspect for molecules to be considered a “good” candidate for vaccine adjuvant is to be able to enhance the efficacy of weak antigens by inducing appropriate immune responses. Another very important aspect is that an adjuvant cannot induce an overwhelming immune response and have a toxic effect. A lot of work still needs to be done to fully understand the types of immune response as well as the mechanism of action of potential nano-adjuvants to further gain knowledge for developing sustainable nanotechnology for vaccines.
These findings are described in the article entitled Mechanisms of the immune response cause by cationic and anionic surface functionalized cellulose nanocrystals using cell-based assays, recently published in the journal Toxicology In Vitro.
References
- Bookstaver, M. L., Tsai, S. J., Bromberg, J. S., and Jewell, C. M. (2018) Improving Vaccine and Immunotherapy Design Using Biomaterials, Trends in immunology 39, 135-150.
- Lahiri, A., Das, P., and Chakravortty, D. (2008) Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond, Vaccine 26, 6777-6783.
- Awate, S., Babiuk, L. A., and Mutwiri, G. (2013) Mechanisms of action of adjuvants, Frontiers in immunology 4, 114.
- Wilson, N. S., Duewell, P., Yang, B., Li, Y., Marsters, S., Koernig, S., Latz, E., Maraskovsky, E., Morelli, A. B., Schnurr, M., and Ashkenazi, A. (2014) Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant, J Immunol 192, 3259-3268.
- Shen, Y., Hao, T., Ou, S., Hu, C., Shen, L. Applications and perspectives of nanomaterials in novel vaccine development (2018) Med. Chem Commun 9, 226–238.
- Sunasee R, Araoye, E., Pyram, D., Hemraz, U.D., Boluk, Y., Ckless, K. (2015). Cellulose nanocrystal cationic derivative induces NLRP3 inflammasome-dependent IL-1β secretion associated with mitochondrial ROS production. Biochem Biophys Rep, 4, 1-9.
- Despres, H.W., Sabra A., Anderson P., Hemraz U.D., Boluk Y., Sunasee R., Ckless, K. (2019) Mechanisms of the immune response cause by cationic and anionic surface functionalized cellulose nanocrystals using cell-based assays. Toxicol In Vitro, 55, 124-133.
- Jimenez, A. S., Jaramillo, F., Hemraz, U. D., Boluk, Y., Ckless, K., and Sunasee, R. (2017) Effect of surface organic coatings of cellulose nanocrystals on the viability of mammalian cell lines, Nanotechnol Sci Appl 10, 123-136.
Acknowledgment: The authors would like to thank InnoTech Alberta for the generous donation of CNCs for our studies. This material is based upon work supported by the National Science Foundation under Grant No 1703890.









