
Many modern products that are sold require highly specialized separation techniques for their purification and preparation. Some of the separations that have become more common are the separations of liquids from solid matrices or mixtures that contain solids. This is particularly the case in the pharmaceutical and natural product industries.
Supercritical fluid extraction, or SCFE for short, makes use of an interesting property of chemicals at high pressures and temperatures, where they have a combination of properties that are usually only exhibited by either liquids or gases. Due to these properties, these chemicals can be used as “super-solvents” for the separation of complex mixtures, because they can dissolve large amounts of solutes while diffusing through a material in a similar manner to a gas. SCFE can, therefore, be used for many applications where solids are present in the complex mixtures and where conventional liquid solvents are not as effective.
At present, most SCFE processes use carbon dioxide as the solvent, because it is cheap, readily available, and relatively harmless. However, despite having these advantages, carbon dioxide does not always provide the best separation, and other chemicals (known as co-solvents) often need to be added with the solvent to enable a good separation. The challenge with the addition of these co-solvents is that they become contaminants in the extracted product. Therefore, in our research we proposed that alternative chemicals, such as fluorinated alkanes, be used in SCFE processes, as these would be capable of performing different separations at similar process conditions without the need for co-solvents.
To identify the solutes for which these alternative solvents would be best suited, and thereafter the process conditions at which the optimal performance could be achieved, we started by performing thermodynamic phase equilibrium measurements. In these measurements, we investigated the relationship between the pressure, temperature, and composition of the various phases of mixtures of the solvent with a solute, present in a closed cell. In our measurements, either two or three phases were present. This was a single vapor (gas) phase together with either one or two liquid phases. The measurements were conducted at a variety of temperatures and pressures, which enabled us to obtain a more complete understanding of the mixture behavior.
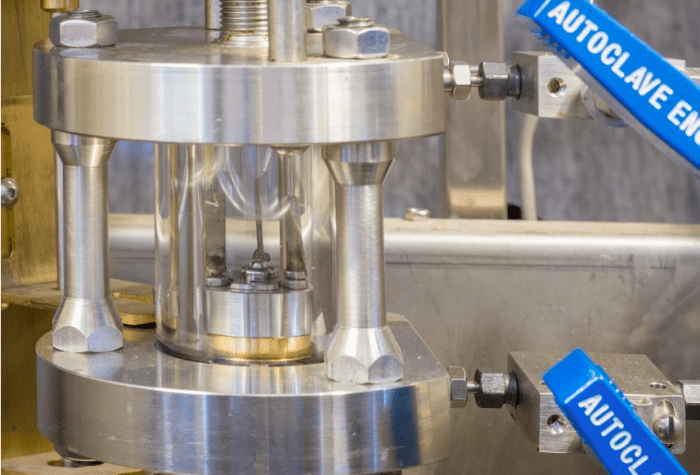
Figure 1. A cell for the measurement of high-pressure phase equilibrium data
The pressures of measurement which were attained in our measurements were as high as 8 MPa. This meant that we required a specially-designed cell, constructed from sapphire crystal, with highly sensitive temperature probes and pressure gauges and a high-pressure sampling mechanism (ROLSI®) attached. The temperature and pressure sensors were highly accurate, with uncertainties in temperature and pressure of as little as 0.05K and 0.005 MPa, respectively. The compositions of the samples that were removed from the cell by the ROLSI® were analysed using a gas chromatograph, which operates by separating the different components and thereafter measuring the amount of each component present individually.
The data that are obtained from the measurements did not provide much insight in their raw format, and therefore thermodynamic models were fitted to the data to make the data more useful. For example, once a model was fitted to the data for a system, this model was used in a process simulation program, such as Aspen Plus, to design a chemical process. The most commonly used model in our research was the Peng-Robinson equation of state, modified with the Mathias Copeman alpha function and utilizing the Wong-Sandler mixing rules, together with the Non-Random Two-Liquid Gibbs’ excess energy activity coefficient model. These complex models attempt to describe the relationship between the temperatures, pressures, and compositions of the systems, and for most of our research, differed from the experimental data by less than 5 %. Because the main aim of this research was to discover the best supercritical solvent for the various solutes, an important aspect of the research was the determination of when the mixture was in the supercritical phase. This was done experimentally, as well as by using the thermodynamic models in several calculation procedures.
In this research,1-9 we have performed phase equilibrium measurements for a number of different mixtures, and this has enabled us to develop processes for the separation of different components from one another, simply by modifying the temperatures and pressures of the processes. Two examples of plots of the measured data, together with the thermodynamic models, are shown in Figures 2 and 3. The black dotted lines on both figures are the critical locus curves, which are the calculated conditions at which the mixture would become a supercritical fluid.
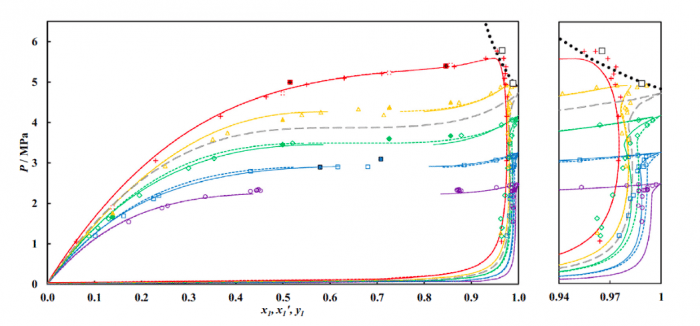
Figure 2. Measured phase equilibrium data, together with several different models for the system of trifluoromethane (1) and n-hexane (2) at temperatures of between (272.87 and 313.20) K.1. Reprinted with permission from Elsevier, the original figure can be found here: https://doi.org/10.1016/j.jct.2017.12.002
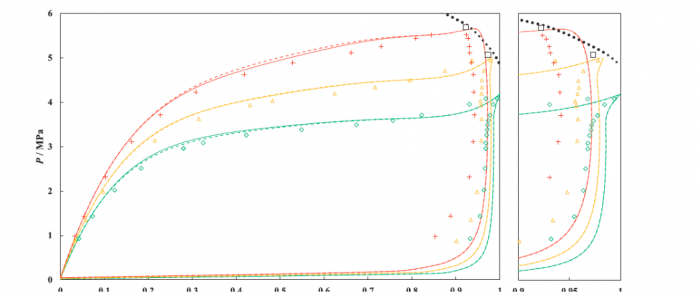
Figure 3. Measured phase equilibrium data, together with several different models for the system of trifluoromethane (1) and 1-hexene (2) at temperatures of between (293.1 and 313.3) K.2. Reprinted with permission from Elsevier, the original figure can be found here: https://doi.org/10.1016/j.jct.2017.12.002
These findings are described in the article entitled Isothermal vapour-liquid equilibrium data for binary systems of (CHF3 or C2F6) with (1-hexene or 3-methylpentane), recently published in the Journal of Chemical Thermodynamics. This work was conducted by Mark D. Williams-Wynn, Paramespri Naidoo, and Deresh Ramjugernath from the University of KwaZulu-Natal.
References:
- Williams-Wynn, M. D.; Naidoo, P.; Ramjugernath, D. Isothermal (vapour + liquid) equilibrium data for binary systems of (n-hexane + CO2 or CHF3). J. Chem. Thermodyn. 2016, 94, 31-42.
- Williams-Wynn, M. D.; Naidoo, P.; Ramjugernath, D. Isothermal vapour-liquid equilibrium data for binary systems of (CHF3 or C2F6) with (1-hexene or 3-methylpentane). J. Chem. Eng. Data 2018, 121, 79-90.
- Williams-Wynn, M. D.; Naidoo, P.; Ramjugernath, D. Isothermal vapour-liquid equilibrium data for the binary systems of (CHF3 or C2F6) and n-heptane. J. Chem. Thermodyn. 2016, 102, 237-247.
- Ramjugernath, D.; Valtz, A.; Richon, D.; Williams-Wynn, M. D.; Coquelet, C. Isothermal Vapor–Liquid Equilibrium Data for the Hexafluoroethane (R116) + n-Butane System at Temperatures from 273 to 323 K. J. Chem. Eng. Data 2017.
- Williams-Wynn, M. D.; El Abbadi, J.; Valtz, A.; Kovacs, E.; Houriez, C.; Naidoo, P.; Coquelet, C.; Ramjugernath, D. Experimental determination of the critical loci for R-23 + (n-propane or n-hexane) and R-116 + n-propane binary mixtures. J. Chem. Thermodyn. 2017, 108, 84-96.
- Ramjugernath, D.; Valtz, A.; Richon, D.; Williams-Wynn, M. D.; Coquelet, C. Isothermal Vapour-Liquid Equilibrium Data for Binary Mixtures of Hexafluoroethane (R116) + n-Pentane or n-Hexane at Temperatures from (288 to 297) K. J. Chem. Eng. Data 2018, 63, 1228-1233.
- Williams-Wynn, M. D.; Naidoo, P.; Ramjugernath, D. Isothermal vapour-liquid equilibrium data for the binary systems of (CHF3 + n-octane) and (C2F6 + n-octane). J. Chem. Thermodyn. 2018, unpublished.
- Williams-Wynn, M. D.; Naidoo, P.; Ramjugemath, D. Isothermal Vapor–Liquid Equilibrium Data for Binary Systems of CHF3 or C2F6 with Methylcyclohexane or Toluene. J. Chem. Eng. Data 2018, 63, 2114-2126.








