
Chemotherapy is widely used to treat cancer patients in the clinic. During administration, toxic chemo-drugs travel through the body, killing cancer cells but also affecting fast-growing healthy cells, including blood-forming cells in the bone marrow, hair follicles, cells in the mouth, digestive tract, and reproductive system. Damage to these healthy cells will eventually cause undesired side effects, like anemia, easy bruising and bleeding, hair loss, nausea and vomiting, appetite changes, constipation and others [1,2].
Stimuli-responsive drug vehicles have been developed to avoid those potential side effects and improve the safety and efficacy of chemotherapy. Controlling drug release under external stimuli (e.g., laser, ultrasound, and electromagnetic field) enables localization of the chemo-drug to the targeted cancer cells. However, most responsive strategies are still not clinically approved or applied to cancer patient treatment, possibly because suitable instruments for producing those external stimuli are not well developed [1].
Cryosurgery (also called cryotherapy or cryoablation) has been clinically applied to treat various diseases, including cancer, by destroying diseased tissues with cooling or freezing [3]. Designing around this clinically used external stimulus, Wang et al., for the first time, have developed cold-responsive nanocarriers to deliver and control the release of drug at the tumor area when the temperature is lowered below ⁓ 10°C [4].
First, a mixture of polymers — pluronic F127 (PF127), poly(N-isopropylacrylamide-co-butylacrylate) (PNIPAM-B), chitosan-modified PF127 (PF127-chitosan), and hyaluronic acid (HA) — was optimized to form the nanocarriers through a simple double emulsion method (Figure 1a). These nanocarriers are stable at room or body temperature with a size of ⁓100 nm, while quickly “melting” at low temperature. This is mainly because of the thermo-responsive capacity of PNIPAM-B. The polymer is hydrophobic at room or body temperature, which can help to maintain the structure of the nanoparticles, but becomes hydrophilic at cold temperature, resulting in irreversible disassembly of the nanostructure (Figure 1b).
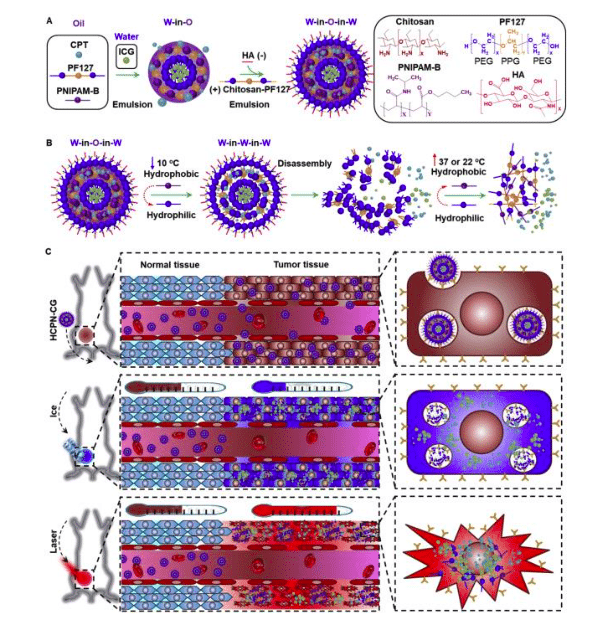
Figure 1. Synthesis and mechanism of cold-responsive nanoparticle for drug delivery to treat cancer. Reprinted from [4] with permission of Elsevier.
Since cancer stem-like cells (CSCs) are posited for the many failures of chemotherapy due to their high capacity of drug resistance, HA was modified on the surface of the nanoparticles for targeted delivery of chemo-drugs to CSCs. HA uniquely binds with the variant CD44, a surface marker that overexpressed on many types of CSCs. This may help to deliver more chemo-drugs into the CSCs to enhance the anti-tumor/CSC capability of the nanoparticles.
To further improve the anti-CSC and cancer cell capability of irinotecan, a small molecular dye, indocyanine green (ICG), was co-encapsulated into the nanoparticles for photothermal warming under near-infrared (NIR) laser irradiation. This photothermal effect, thermal energy (heat) produced by excitation of the dye, could enhance the cytotoxicity of chemotherapeutic drugs [5]. Moreover, severe damage to the cancer cells/CSCs morphology was observed by combining both cooling and warming.
This concept is further confirmed by in vivo animal studies. Tumor-bearing mice were first treated with nanoparticles through intravenous injection. After 12 hours, tumors were cooled with ice and then irradiated with NIR laser (Figure 1c). This combination technology exhibits the best anti-tumor capacity, drastically shrinking the tumors and lowering the percentage of CSCs in the mice comparing to all other control groups.
The work is significant as it combines new, innovative nanomedicine with existing cancer treatment, which is important for potential clinical application. The excellent anti-tumor capacity of this method shows great promise for safer and more effective cancer treatment. Moreover, the team is investigating more applications of these novel cold-responsive nanocarriers, such as cell and tissue banking.
These findings are described in the article entitled Enhanced cancer therapy with cold-controlled drug release and photothermal warming enabled by one nanoplatform, recently published in the journal Biomaterials. This work was conducted by Hai Wang, Pranay Agarwal, Yutong Liang, Jiangsheng Xu, Gang Zhao, Katherine H.R. Tkaczuk, Xiongbin Lu, Xiaoming He from the University of Maryland, Ohio State University, University of Science and Technology of China, and Indiana University School of Medicine.
References:
- M.A.C. Stuart, W.T. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G.B. Sukhorukov, I. Szleifer, V.V. Tsukruk, M. Urban, Emerging applications of stimuli-responsive polymer materials, Nature materials 9(2) (2010) 101.
- N. Carelle, E. Piotto, A. Bellanger, J. Germanaud, A. Thuillier, D. Khayat, Changing patient perceptions of the side effects of cancer chemotherapy, Cancer 95(1) (2002) 155-163.
- A.A. Gage, Cryosurgery in the treatment of cancer, Surgery, gynecology & obstetrics 174(1) (1992) 73-92.
- H. Wang, P. Agarwal, Y. Liang, J. Xu, G. Zhao, K.H. Tkaczuk, X. Lu, X. He, Enhanced cancer therapy with cold-controlled drug release and photothermal warming enabled by one nanoplatform, Biomaterials 180 (2018) 265-278.
- H. Wang, P. Agarwal, S. Zhao, J. Yu, X. Lu, X. He, A biomimetic hybrid nanoplatform for encapsulation and precisely controlled delivery of theranostic agents, Nature communications 6 (2015) 10081.








