
The quest for two-dimensional (2D) materials, starting with graphene in 2004, remains strong. While graphene retains the title of strongest solid, intense research has shown that it is not suited for every use. For example, turning off the current flowing through graphene transistors has proven quite a challenge. Moreover, high-quality graphene lacks pores, which prevents it from achieving certain applications.
By contrast, it is possible to change pore sizes in other 2D materials simply by stretching them. This enables one to control the permeability of 2D membranes to various gases, liquids, and ions mechanically. This effect could, for example, be used to desalinate water or separate gases. Single layers of COF-1 are an example of a porous 2D material, belonging to the Covalent Organic Framework family of organic solids.
In a recent publication, authors from Texas and Brazil found another trick that can be played in this material: elongate COF-1 enough, and its adhesion goes down. The reported results were obtained by combining classical and quantum computer simulations.
The interplay between strain and adhesion in COF-1 stems from structural changes caused by the external tension. Before explaining this effect, however, it is important to recall some key attributes. First, note that pulling a solid along one direction often causes it to contract in transverse directions, as seen when one stretches a rubber band. Second, notice in figure 1a that the atomic structure of COF-1 is rather peculiar, being composed of large porous rings that, in turn, are comprised of smaller rings linked by single bonds.
This information can be used to qualitatively understand the response of COF-1 to mechanical deformation. When strain is low, the material easily shrinks laterally through pore size reduction. However, pore reduction eventually brings atoms into contact, leading to repulsion, which hampers lateral contraction. For even larger strain values, it is possible to limit repulsion while allowing for lateral contraction by partially displacing atoms outside of the initial plane, through ring rotations. Indeed, if the external strain is greater than 15%, the optimized COF-1 structure is non-planar, see figure 1b.
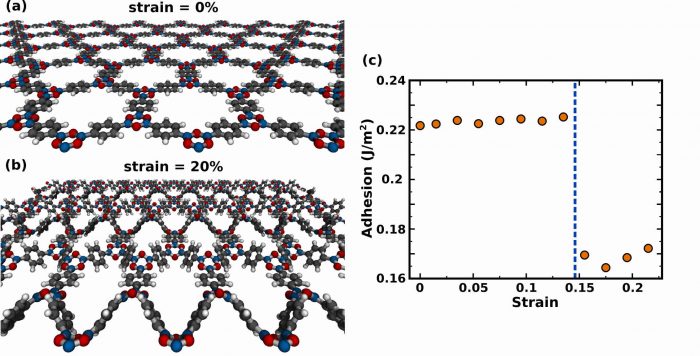
Figure 1: Snapshots of the atomic structure of COF-1 under strain are presented in (a) and (b). Notice that for an elongation of 15% the optimized structure is no longer planar. (c) Graph of adhesion as a function of strain. Notice the sharp decline associated with the transition from planar to non-planar. Figures courtesy Leonardo Machado.
Finally, recall that the attraction between COF-1 and other materials arises from non-bonded interactions at their interface, and so adhesion increases with contact area. At large strain values, the rugged non-planar structure of COF-1 hinders contact with other materials, reducing adhesion – see figure 1c. One important trait of this structural change is that it preserves all chemical bonds. Hence, removing tension restores COF-1 to its planar form.
To illustrate a possible use for the strain-controlled adhesion, the authors reported additional classical molecular dynamics simulations, where they used the effect to move a piece of graphene. In these lower accuracy calculations, adhesion increased with strain instead of decreasing (as in the more precise computations). Still, the concept should work as long as adhesion changes with elongation. The movie below condenses the molecular dynamics results.
The above trajectory illustrates the possibility of moving 2D materials via the mechanical control of adhesion. In the macroscale, mechanical stimuli are often used to move small objects as well – think of tweezers. In this sense, single layers of COF-1 act as nanoscale tweezers, picking up and dropping off atomically thin solids through selective strain application.
These findings are described in the article entitled Prediction of strain-controlled adhesion in a single-layer covalent organic framework, recently published in the journal Carbon.









