Until the early 2000s, nanotechnology was only considered as a means to manipulate matter at the atomic or molecular level. Since then, there has been a rise in advancement in this field allowing scientists to widen the scope of possibilities to different biomedical as well as industrial fields (1). Nanoparticles (NPs), because of their unique structural and functional properties, have flourished in these sectors compared to their bulk counterparts.
Nevertheless, there is also an ongoing debate on the issue of undesirable toxic outcomes of NPs when interacting with living systems. As the key organizer controlling the entire physiological activities of the living system, the Central Nervous System (CNS) must be emphasized primarily in the perspective of nanotoxicity (2). One of the major challenges met by researchers in the 21st century is the concern about the ability of NPs to cross the blood-brain barrier (BBB). This further elevates the possibility of NP-induced toxic responses in neuronal cells. Studies claim that NPs are often able to disrupt neuronal cell membrane with the utmost severity. For example, in 2007, Sharma et al. showed that NPs including Al, Cu, or Ag administered via intravenous, intraperitoneal, and intracerebral routes in experimental rats induced BBB disruption (3).
Researchers accomplished a lot concerning the general toxicity mechanism of NPs in a range of tissues. However, there is a blank in elucidating the specific molecular toxicity mechanisms exhibited by nanoparticles by means of the CNS. According to the CNS toxicity studies performed so far, NPs have proven to cause pathophysiological complications within various cells and structures of CNS. As made obvious by the observations in other biological tissues, one of the foremost toxic responses elicited by NPs in CNS includes the generation of reactive oxygen species (ROS) and oxidative stress. This is found to be applicable in the case of particulate matter (PM), wherein NPs occupying active moiety can raise the levels of ROS in neuronal tissue with much prevalence (4). This ROS would then worsen the circumstance by damaging major metabolic mediators including proteins, nucleic acids, lipids, etc., specifically at the deposition site.
Following draining into circulation promotes translocation to other body tissues, hence extending the harmful outcomes of oxidative stress. The dreadful consequence lies in the fact that due to the extremely high demand of energy, lower levels of antioxidants, and higher levels of oxidative stress, targets in the brain have turned itself into a more vulnerable tissue. Prominent NPs leading to the production of such ROS include Carbon nanotubes (CNTs), C60 fullerenes, transition metal oxide quantum dots, etc.
Most psychiatric disorders are reported to be linked with NP-induced interruption of the Electron Transport Chain (ETC) in mitochondria, thereby leading to oxidative stress (5). NPs vary widely in their ability to induce cytotoxic responses in the brain in accordance with their physico-chemical and functional properties. In particular, Hardas et al. observed in 2010 that administration of ceria NP alters the expression of phase-II proteins in the hippocampal region of a rat. This further disrupted the antioxidant enzyme level in the brain region with severe oxidative stress. Moreover, since these enzymes play crucial roles in the protection of the tissue from the invasion of mutagens and other foreign entities, the immune safety of the animal was extremely compromised (6).
Microglia represents the primary line of defense mechanism in CNS which undergoes significant morphological and functional alterations upon NPs’ way in. Silicon NP induced reduction in the percentage of glial cell community levels and functional deprivation was previously reported by Choi et al. in 2009 (7). Overexpression of pro-inflammatory mediators and cytokines levels is also possible during NP interaction with CNS. Regarding autophagic degradation of neuronal cells and cellular debris, NP was reported to be a causative agent for altered expression genes involved in this cycle and, hence, can induce undesirable apoptotic events. This adds possibilities for dreadful neurodegenerative disorders like Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, etc (8). A diagrammatic representation of NP-induced cytotoxic responses in neuronal cells, in general, is given in figure 1.
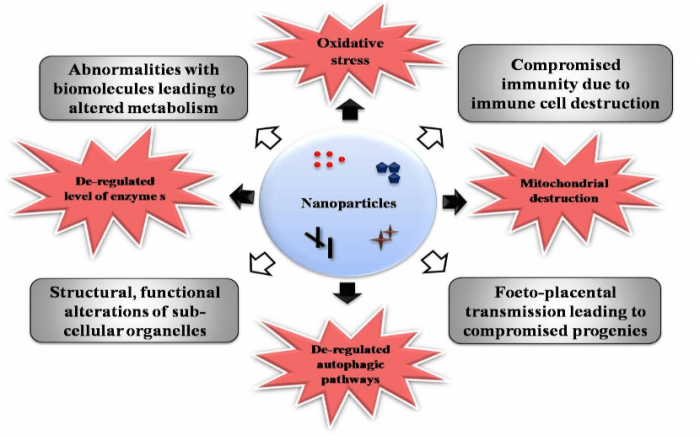
Figure 1. Nanoparticle-induced toxic responses in neuronal cells in general. Figure courtesy Mohanan PV.
The present review addresses the consequences of NP interaction with CNS with more emphasis on cellular and molecular level toxic responses in neuronal cells. The review begins with an accent on different pathways for NP entrance into CNS like the BBB pathway, olfactory pathway, and placental barrier pathway. Afterward, a detailed account of the potent cellular toxicity mechanisms exhibited by NPs including oxidative stress, mitochondrial deformities, subsequent abnormalities within immunological functions, as well as de-regulated autophagy pathways and apoptosis will be highlighted. A brief note on various factors affecting NP toxicity in CNS is portrayed as well. In addition, emerging possibilities of employing NPs for diverse diagnosis applications like neuronal cell imaging, stem cell migration studies, as CNS sensors, etc., will also be concisely reviewed. The review ends by highlighting the requirement for evaluating the possible role of NPs in the onset of neurological abnormalities. An overall illustration of the major highlights of the review is depicted in Figure 2.
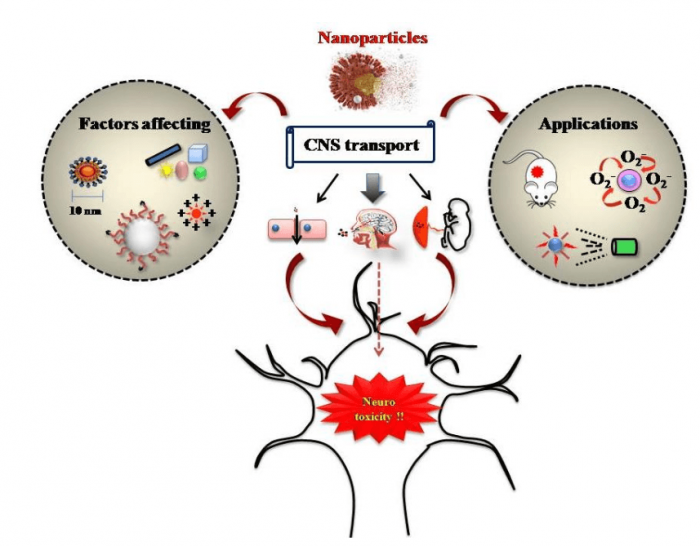
Figure 2. The major highlights of the review (principal routes of nanoparticle entry into CNS, cellular toxicity mechanisms, factors affecting nanoneurotoxicity, and application of nanoparticles for CNS disease diagnosis and curing). Image courtesy Mohanan PV.
In nutshell, the review opens up proper guidance to researchers regarding the neurotoxic potential of NPs and how they lend a hand in adopting useful strategies for nanomedicine-based medical purposes.
These findings are described in the article entitled Interaction of nanoparticles with central nervous system and its consequences, recently published in the American Journal of Research in Medical Sciences, 4(1): 12-32. The work was done by Athira SS, Prajitha N and Mohanan P V from Sree Chitra Thirunal Institute for Medical Sciences and Technology (Govt. of India), Trivandrum, Kerala, India.
References:
- Nikalje, A.P., 2015. Nanotechnology and its applications in medicine. Med chem, 5(2): 081-089.
- Win-Shwe, T.T. and Fujimaki, H., 2011. Nanoparticles and neurotoxicity. International journal of molecular sciences, 12(9): 6267-6280.
- Sharma, H.S. and Sharma, A., 2007. Nanoparticles aggravate heat stress induced cognitive deficits, blood–brain barrier disruption, edema formation and brain pathology. Progress in brain research, 162: 245-273.
- Dellinger, B., Pryor, W.A., Cueto, R., Squadrito, G.L., Hegde, V. and Deutsch, W.A., 2001. Role of free radicals in the toxicity of airborne fine particulate matter. Chemical research in toxicology, 14(10): 1371-1377.
- Bressan, E., Ferroni, L., Gardin, C., Rigo, C., Stocchero, M., Vindigni, V., Cairns, W. and Zavan, B., 2014. Silver nanoparticles and mitochondrial interaction, Neurotixicology: 32-43.
- Hardas, S.S., Butterfield, D.A., Sultana, R., Tseng, M.T., Dan, M., Florence, R.L., Unrine, J.M., Graham, U.M., Wu, P., Grulke, E.A. and Yokel, R.A., 2010. Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicological sciences, 116(2): 562-576.
- Choi, J., Zheng, Q., Katz, H.E. and Guilarte, T.R., 2009. Silica-based nanoparticle uptake and cellular response by primary microglia. Environmental health perspectives, 118(5): 589-595.
- Nixon, R.A., 2013. The role of autophagy in neurodegenerative disease. Nature medicine, 19(8): 983.









