
The development of new materials using natural polymers provides an opportunity to meet industry demand and at the same time mitigate adverse environmental impacts associated with the overuse of petroleum-derived plastics.
The challenge of rationally designing such polymers and the scalability of their production can be overcome by combining a biomimetic “mix-and-match” approach of creating hybrid molecular structures with capabilities which enable the “instructing” of plants to make such polymers using biosynthesis. The idea of how this can be enabled comes from a genus of plants called Plantains, members of which are “cosmopolitan” weeds ubiquitously distributed across all continents (Figure 1).

Figure 1. Illustration of different species of Plantago genus. The Images of plants are reproduced with permission from The Seeds of South Australia http://saseedbank.com.au/.
Seeds of these plants produce a jelly-like material called mucilage, which comes in a variety shapes and forms. The unique feature of the constituent polymers is their brush-like or, better put, “pipe cleaner-like” structure. While most polymers including those in plastic materials are “spaghetti-like,” Plantago polysaccharides have a backbone decorated with side-chains (Figure 2).
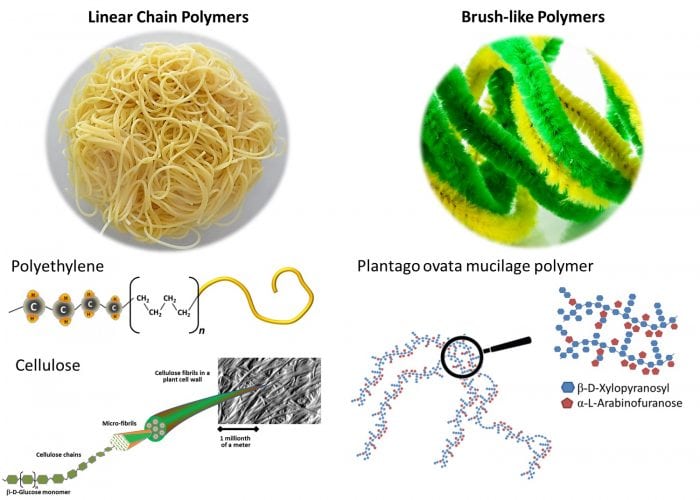
Figure 2. Illustration of different types of polymers, including unique brush-like polymers from Plantago. Credit: Gleb Yakubov
Recently, it was established that the composition, distribution, and repeated motif of these side-chains determine physical and material properties of these molecules [1]. Different species of Plantains “utilize” these side-chain decorations as a “mix-and-match” toolbox for creating a variety of chemical structures based on a relatively simple template.
The side-chain decorations appear to be a simple way of creating multi-functional polymers using only a relatively small number of functional groups. Thus, gel-like mucilage of Plantago ovata comprises complex arabinoxylans; the backbone of these molecules is a xylan chain (approximately 3000 units long) decorated by side chains that comprise both xyloses and arabinoses.
Without decorations, xylan is totally insoluble in water, but upon decoration, typically with the sugar called arabinose, becomes fully water soluble. Plantago took this opportunity further and evolved complex biosynthesis mechanisms that put decorations that comprise a mixture of xylose and arabinose residues to tune the properties of their gels from very weak and flowable to those that are tough and resistant to drying.
One of the key drivers of interaction in polysaccharide systems is hydrogen bonding that stems from the ability of hydrogen and oxygen atoms of different molecules to attract to each other. The presence of decorations can control such molecular interactions, and it is possible to introduce “sticky” groups which can form strong physical bonds and lead to the formation of polymer networks. Surprisingly, this property can be amplified by introducing certain motifs or repetitions, which enable to amplify weak interactions and produce a large, macroscopically significant effect.
It is envisaged that discovery of motif-dependent interactions will open new opportunities for the rational design of polysaccharides with targeted and highly-tuned physical properties, providing a far-reaching potential for delivering novel tailor-made hydrocolloids for use in foods, the pharmaceutical industry, and smart materials.
A word on materials, plastics and polymers
Materials were always defining human history (stone age, bronze age, iron age, etc.). The 20th century became known as the age of plastics. Plastics are materials made of polymers, complex molecules formed by repeating a chemical unit tens of thousands or even millions times over.
The unique properties of plastics are largely associated with the fascinating properties of polymers — the ability to entangle like spaghettis in a bowl. These entanglements provide physical strength, while retaining flexibility, extensibility, and the ability to plasticise and form different shapes, which is a hallmark of plastic materials.
The widespread use of petroleum-derived plastics resulted in major problems of plastic recycling, including the presence of microscopic plastic particles in the oceans, which accumulate in marine animals and plants, leading to grave consequences for marine ecosystems. Replacement of petroleum plastics with a plant-derived polymer can resolve these issues and can form a long-term basis for further economic development.
A word on cellulose
Out of many natural materials, the one that is still very much in use is cellulose, of which wood, paper, and textiles such as cotton, linen, and even semi-synthetic materials, such as viscose, are made. Despite a unique set of properties, application and use of cellulose-based materials is reactive and lacks the level of tuning required and expected of modern materials to suit a broad variety of applications. Extending our portfolio of natural polymers, such as those found in the Plantago genus, can complement and enhance our use of cellulose-derived materials and bridge the gap currently filled with petroleum-based plastics.
These findings are described in the article entitled Rheological and structural properties of complex arabinoxylans from Plantago ovata seed mucilage under non-gelled conditions, recently published in the journal Carbohydrate Polymers. This work was conducted by Long Yu, Gleb E. Yakubov, and Jason R. Stokes from the University of Queensland, and Marta Martínez-Sanz and Elliot P. Gilbert from the Australian Nuclear Science and Technology Organisation.
References:
- Yu, L., Yakubov, G. E., Zeng, W., Xing, X., Stenson, J., Bulone, V., & Stokes, J. R. (2017). Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydrate Polymers, 165, 132-141
- Yu, L., Yakubov, G. E., Martínez-Sanz, M., Gilbert, E.P., & Stokes, J. R. (2018). Rheological and structural properties of complex arabinoxylans from Plantago ovata seed mucilage under non-gelled conditions. Carbohydrate Polymers, 193, 179-188.
- Yu, L., Yakubov, G. E., Gilbert, E.P., & Stokes, J. R. (2018). Multi-scale assembly of hydrogels formed by highly branched arabinoxylans from Plantago ovata seed mucilage studied by USANS/SANS and rheology. Carbohydrate Polymers, in press.








