
Nerve cells have long processes that connect one cell to the other, orchestrating nerve-cell networks and allowing the brain to function. A major brain protein called Tau is involved in facilitating dynamics and structure within the nerve cells. In dementia and many neurodegenerative diseases, Tau undergoes structural changes (becomes overly phosphorylated) and precipitates inside cells in structures known as neurofibrillary tangles. Dr. Alzheimer originally discovered these structures when he analyzed the brain of his first Alzheimer’s disease patient.
Tau is a product of a single gene, but once transcribed into RNA and then translated into a protein, it can form a dynamic Tau (three binding sites to the cellular skeleton and transport system – in short, 3R) or a stable Tau with an additional binding site (4R). In Alzheimer’s disease, the neurofibrillary tangles present 3R and 4R Tau forms. In contrast, there are pure 4R Tau pathologies like progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD).
In our recent paper cited below, we show differential phosphorylation of Tau 4R and Tau 3R, which affect interaction with the cellular skeleton, thus affecting brain plasticity.
We further introduce NAP (davunetide, CP201) a drug candidate that enhances nerve cell plasticity, as preferring the 3R Tau and fortifying cell skeleton and transport system dynamics, thus preferably protecting against 3R or mixed Tau pathologies. These results unravel the precise molecular interactions that explain why NAP (davunetide, CP201) was not efficacious in PSP patients (4R Tau pathology) but showed efficacy in elderly patients suffering from amnestic mild cognitive impairment (3R+4R Tau pathology). The results further suggest the protection of brain developmental processes involving 3R Tau.
NAP is an active small fragment of an activity-dependent neuroprotective protein (ADNP). Children suffering from the ADNP syndrome (mutation in the ADNP gene) are characterized by global developmental delays, intellectual disabilities, and autistic traits, which can now be explained in part by deregulated interaction with 3R Tau, offering hope for CP201 development for the ADNP children.
Coronis Neurosciences (Professor Gozes, Chief Scientific Officer) is developing CP201 for the ADNP syndrome (US FDA, orphan drug designation).
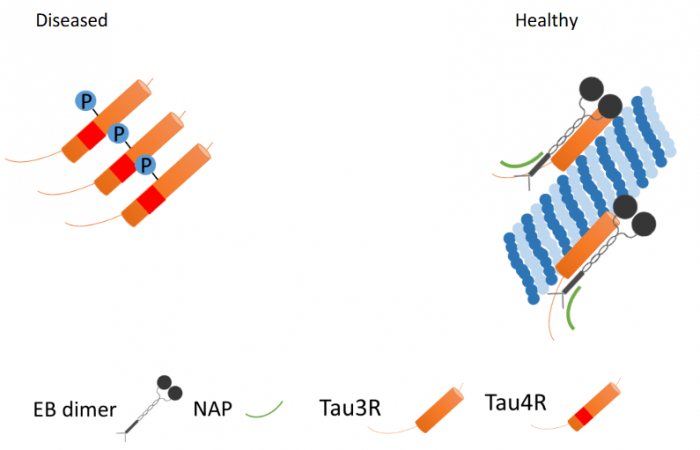
Proteins keeping the nerve cell alive: Microtubules constitute the brain cell scaffold and transportation system within the cell. EB – microtubule end-binding proteins enhance Tau-microtubules connection, which is further enhanced by NAP (davunetide, CP201). P – phosphate. NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies. Credit: Illana Gozes
These findings are described in the article entitled NAP (davunetide) preferential interaction with dynamic 3-repeat Tau explains differential protection in selected tauopathies, recently published in the journal PLOS One.
Citation:
- Ivashko-Pachima Y, Maor-Nof M, Gozes I. PLoS One. 2019 Mar 13;14(3):e0213666. doi: 10.1371/journal.pone.0213666. eCollection 2019.









