
The microbial electrolysis cells are innovative technologies that utilize “electroactive microorganisms” as biocatalysts into an electrochemical device; these electroactive microorganisms can exchange electrons with a conductive material through the external electron transfer mechanism, using the conductive material as electron donor or acceptor.
The current generation from a microorganism’s metabolism was firstly reported in 1911 by M.C. Potter in his scientific communication letter “Electrical Effects Accompanying the Decomposition of Organic Compounds”1, where he stated that:
The results in electrophysiology have familiarised us with the view that any physiological process accompanied by chemical changes involves an associated electric change M.C. Potter, 1911.
Studies on the interaction between microorganisms and solid materials continued mainly in the field of biocorrosion of metals, especially under marine environment, while in the last 20 years the scientific interest on electroactive microorganisms has been mainly focused on their application in bioelectrochemical systems in the environmental field.
The most well-known bioelectrochemical device developed during the latter period is the air-cathode Microbial Fuel Cell (MFC), which permit to generate electrical energy by coupling the bio-anodic oxidation of organic compounds and the reduction of the oxygen in the air, giving the possibility to directly convert the chemical energy contained in the wastewater into electrical energy. Even if the MFC energy outputs are far from the benchmark technologies, the study on MFC led to a new and extremely active research field that today involves more than 900 scientists from all over the world, most of them belonging to the International Society for Microbial Electro Technologies (ISMET).
Nowadays, the bioelectrochemical systems are investigated for a wide number of applications such as: the production of target molecules coupled with wastewater treatment (Microbial Electrolysis Cell, MEC), the desalination of brackish and sea water (Microbial Desalination Cell, MDC) or the direct CO2 fixation into volatile fatty acids (Microbial Electrosynthesis Cell, MES)2. The MECs are the most investigated devices because they can represent a potential strategy to combine the CO2 fixation with the energy storage and the recovery of nutrients.
Credit: Marco Zeppilli
The possibility to use the bioelectrochemical methanation as a strategy for the use of electricity overproduction, especially from renewable sources, has been proposed in the bioelectrochemical power-to-gas concept (BP2G)3 where the concentrated CO2 streams like the biogas or other flue gases can be converted into methane by using a methane-producing biocathode. Moreover, the energy generated by bioelectrochemical reactions permits the concentration and the recovery of ammonium nitrogen from wastewaters, thanks to its migration through a cation exchange membrane. The ammonium recovery represents an important environmental goal because its fixation (performed by the energy-intensive Haber-Bosh process), and removal from wastewaters (nitrification/denifitrication process) are energy intensive processes.
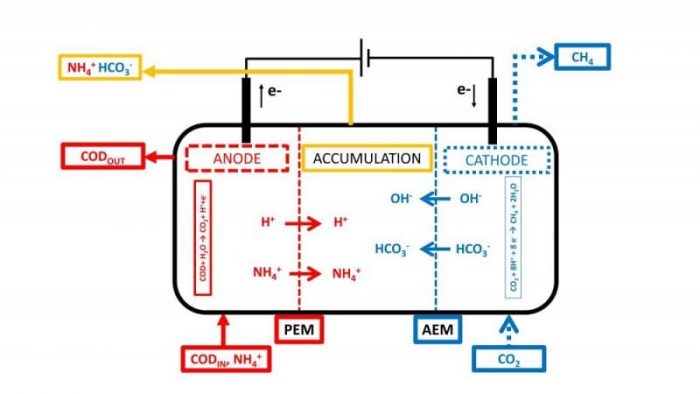
In this context, the study recently published by our research group “Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery”4 reports the performances of an innovative configuration of a Microbial Electrolysis Cell aimed to combine the cathodic methane formation and the CO2 removal with the anodic wastewater treatment, together with the ammonium and bicarbonate recovery. The innovation produced in this publication is the addition of an intermediate chamber, named accumulation chamber, separated by a proton exchange membrane and an anion exchange membrane between the anodic and the cathodic chamber.
Due to the cell configuration, the electroneutrality maintenance was ensured by the migration of cations (ammonium) from the anode to the accumulation chamber and anions (bicarbonate) from the cathode to the accumulation chamber. The driving force for the ion migration is the electricity generated by the anodic and cathodic bio-electrochemical reaction, i.e. the anodic oxidation of organic matter (COD) and the cathodic CO2 reduction; the resulting process acts like a conventional electrodialysis process with the bioelectrochemically generated energy. The main ionic species determined during the operation of the MEC were the ammonium fed by the anodic influent (that simulate a municipal wastewater) and the bicarbonate derived from the CO2 sorption in the cathodic chamber due to the alkalinity generation. The alkalinity generation in the cathodic chamber – the main CO2 removal mechanism – derived from the ionic migration of species different from protons and hydroxyl, directly linked to the current that flowed in the circuit.
The three-chamber MEC process can be potentially used as a post unit treatment for the increase of by-products quality from the anaerobic digestion process, i.e. the biogas can be treated in the cathodic chamber and by the CO2 removal transformed in biomethane, while in the anodic chamber the digestate – that typically contains residual COD and a high concentration of ammonium – partially sustains the energy demand of the process by the residual COD oxidation. Finally, the current generated by the two bioelectrochemical reactions promote the migration of ammonium and bicarbonate, obtaining a concentrated stream in the accumulation chamber that could be utilized as a fertilizer or for other biotechnological purposes like a nutrient solution.
References
- Potter, M. C. (1911). “Electrical Effects Accompanying the Decomposition of Organic Compounds.” Proceedings of the Royal Society of London B: Biological Sciences 84(571): 260-276.
- Schroder, U., F. Harnisch and L. T. Angenent (2015). “Microbial electrochemistry and technology: terminology and classification.” Energy & Environmental Science 8(2): 513-519.
- Geppert, F., D. Liu, M. van Eerten-Jansen, E. Weidner, C. Buisman and A. ter Heijne (2016). “Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives.” Trends in Biotechnology 34(11): 879-894.
- Zeppilli, M., A. Mattia, M. Villano and M. Majone “Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery.” Fuel Cells
The study, Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery was recently published in the journal Fuel Cells.









