
Lead, like many other metals (do not call them “heavy metals”; see Pourret, 2018), is a global pollutant. Lead poisoning, also known as saturnism, occurs most often by ingestion of the metal. The main cause of ingestion of lead is ingestion of dust and paint chips containing lead. This is especially worrying for small children.
Hundreds of tons of lead in Notre Dame’s spire and roof melted during the April 15 fire, coming close to destroying the cathedral, according to several news agencies this month (August 2019). The Paris regional health agency reported that lead concentrations remain elevated at some spots inside the building and in the soil of the adjacent park and forecourt, which have been closed to the public since the fire. But we read and hear much nonsense in the media.
In toxicology, speciation of chemical elements is largely ignored; elements other than carbon are often judged as toxic because of evidence relating toxicity to only a few of the chemical species in which they occur. Since their physical, chemical, and biological characteristics depend on molecular structure and not its elemental constituents, so does its toxicity. Indeed, the toxicity of often-called “toxic” elements, like lead, depends on their speciation and concentration not only in a quantitative way but also in a qualitative way. Therefore, it is essential that toxicological studies always consider the species present rather than elemental constituent in order to create meaningful data. It thus becomes clearer that failure to properly consider chemical speciation of elements can lead to poor risk assessment and bad use of legislation. Laws and regulations based on simple elemental analysis may wrongly consider environmental media or products as toxic.
Speciation analysis provides the information necessary to describe the effects of active species which is not available from the results of total chemical element determinations. The additional information is very useful to direct actions like reducing health risks associated with toxic species.
When it is not possible to determine the concentration of the different individual chemical species that sum up the total concentration of an element in a given matrix, that means it is impossible to determine the speciation. When this happens, it is a useful practice to do fractionation instead. Fractionation is the process of classification of a chemical element from a certain sample according to physical (e.g. size, solubility) or chemical (e.g. bonding, reactivity) properties in the laboratory or using models.
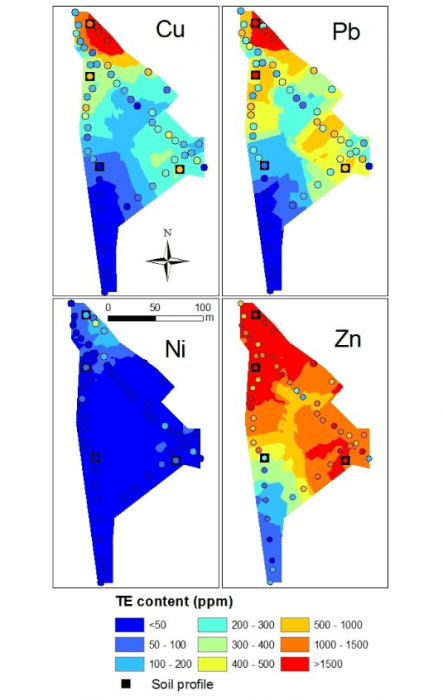
Figure 1 Spatial distribution maps of copper, nickel, lead and zinc and position of the four soil profile studied (■). Figure courtesy Olivier Pourret
A wide knowledge of soil geochemistry is necessary to determine the impact of anthropogenic activities and resulting toxicities. It is widely recognized in the scientific community (but not so much by the general public) that the environmental impact of lead is not dependent on the total soil content. Indeed, it is mediated through dissolved species. Thus, to evaluate lead bioavailability and its environmental impact, we need to look at the chemical speciation. Lead occurs in soil solutions as free ions, or as inorganic or organic complexes bound to various ligands. Lead speciation depends on its pH, its concentration, and ligand concentrations. Among numerous ligands, dissolved organic matter can strongly bind lead. Dissolved organic matter is a complex mixture including reactive humic substances (mostly humic acids and fulvic acids).
To describe the electrostatic and specific interactions between humic substances and metals like lead, numerous models have been developed. Among the most reliable and well-tested models are WHAM (with sub-model Model VI) and ECOSAT (with sub-model NICA-Donnan). One of the key uncertainties using these models is whether the humic substances considered in the modeling are truly representative of the soil solution’s dissolved organic matter. To describe the interactions of dissolved organic matter with lead using these models, the fraction of active dissolved organic matter (i.e., the proportion of dissolved organic matter that can effectively complex with metals) and its composition (relative proportions of humic and fulvic acids) is needed.
We thus made and tested assumptions on these two parameters. However, the proportions of humic and fulvic acids in dissolved organic matter, as well as their concentrations, depends on the type of organic matter and can thus vary from one soil to another. In calcareous soils (as in our case study), the presence of carbonates and the alkaline pH strongly influence lead speciation and its mobility.
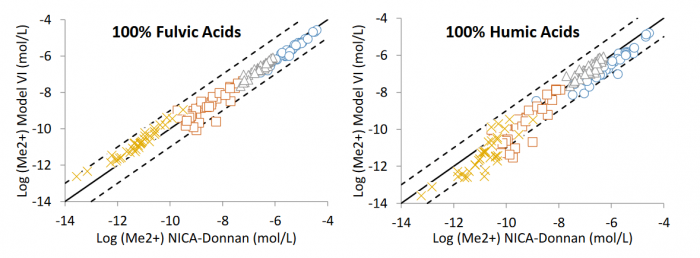
Figure 2 Comparison of free metal ion concentrations (□ copper, ∆ nickel, × lead, ○ zinc) calculated with NICA-Donnan and Model VI expressed in logarithm (modified after Ponthieu et al., 2016). Figure courtesy Olivier Pourret
The objectives of our study published in the Journal of Geochemical Exploration (Ponthieu et al., 2016) were (i) to compare lead speciation results in soil solution obtained by two distinct models, and (ii) to assess the impact of the dissolved organic matter composition on lead speciation. After the evaluation of lead mobility in alkaline soils using partition coefficients, its speciation in the soil solution was evaluated using Model VI and NICA-Donnan with various proportions of humic and fulvic acids. The speciation of lead among other metals (i.e. copper, nickel, and zinc) in the soil solution of 36 samples characterized by alkaline pH and different contamination levels has been determined (Figure 1).
Four assumptions about dissolved organic matter composition have been tested. The results obtained with the two distinct models are of the same order of magnitude (Figure 2). The main result of this study is that the free lead concentrations (i.e. toxic) calculated with the two models may differ by one order of magnitude depending on whether humic and/or fulvic acids are used to represent dissolved organic matter. This work demonstrates the impact of the assumptions made about the dissolved organic matter nature for lead speciation modeling.









