
In an age where dogs and cats live as members of the family and the pet industry is a market worth billions, the wild precursors of these domestic species are facing the most difficult period of their existence. As the newly-baptized Anthropocene is underway, so is another mass extinction event that extends to many species of the world’s biodiversity, but with a particularly serious impact on wild cat species.
This trend has already been observed by experts in animal reproduction science since the 1970s when conservationists began to study and develop biotechnologies of reproduction to support wild species. Given the complex scenario of climate change and relentless numbers (today, there are 3% of the number of tigers that existed in 1900/Living Planet Report 2014 WWF) something has to be done. All cat species are to some degree endangered (IUCN Red List). Biotechnology has become a key element in facing the immense challenge of protecting the world’s biodiversity. The Noah’s Ark of the 21st century is in full construction.
The vision for this set of research efforts to develop reproductive biotechnology is that one day, these techniques would allow us to manipulate the gametes of a species, freeze them, produce embryos, and eventually freeze or transfer these embryos to recipient females. A lot already has been done, but some techniques are still in the improvement phase. Regarding wild cat males, great advances have been made, and today it is possible to freeze millions of sperm, thaw them, and use them for fertilization. There are still many frozen samples from animals that died decades ago.
In the case of females, the situation is completely different. Freezing and being able to manipulate and use large numbers of female gametes, the primordial ovarian follicles remains a scientific challenge. This means that we only have the XY side of the embryos, and we can not keep the XX material, the gametes from the females. The protocols previously developed for the freezing of the ovarian cortex tissue using the slow freezing technique still do not have expressive results and require the use of expensive and sensitive equipment, restricting its applications in many regions.
Based on these premises, we add a field of reality, which in Brazil includes large distances, bad roads, and zoos that are ill-equipped to preserve this material after animal death and, in many places, are far from research centers. At the same time, it is necessary to evaluate the impact of the transport time of this material under the quality required for the frozen primordial ovarian follicles. In summary, it was imperative to develop a protocol that would allow the collection of ovaries of wild feline females that die in different regions of Brazil and safely transport them to research centers. It was also urgent to improve or adapt a freezing protocol for this material which would be feasible even in very basic laboratories where this material could be prepared, glazed, and stored in liquid N2 for future use.
The idea was to try to solve the problem of female gonadal transportation and cryopreservation of the ovarian cortex tissue from felines. The methodology had to simulate the cat ovary transport for long distances (up to 24 hours at 5ºC), before dissecting the ovarian cortex and vitrifying small pieces of tissue full of ovarian preantral follicles, and, after thawing them, recovering many live preantral follicles.
With CNPq scholarship and under the guidance of Professor Dr. Maria Denise Lopes, one of the pioneers with research on wild feline reproduction in Brazil, we were able to establish a partnership between FMVZ/UNESP of Botucatu and the Berlin Zoo IZW Leibniz Institut, and we were able to approve another Scholarship for a Sandwich Doctorate through the Science Without Borders program. I flew to Berlin in March 2013 to develop research under the supervision of Dr. Katarina Jewgenow, one of the most eminent scientists of feline reproduction.
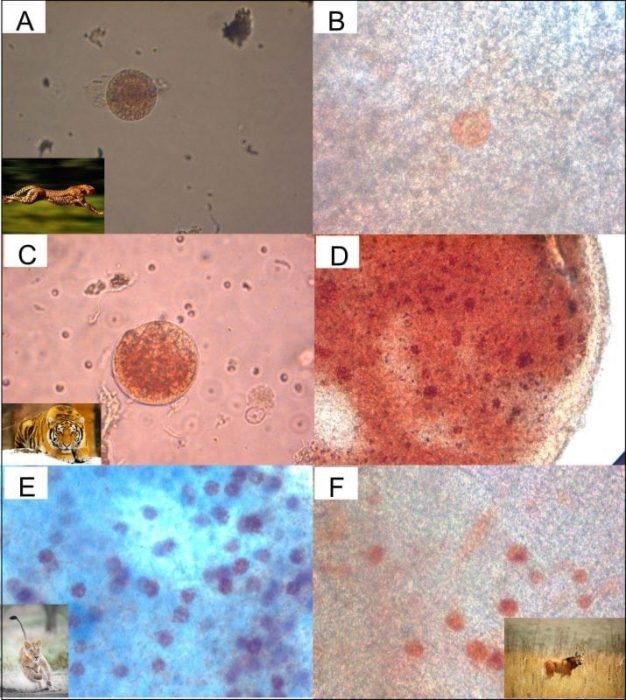
A: Cheetah isolated preantral folicle and B preantral folicle in cortex tissue
C: Tiger isolated preantral folicle and D preantral folicle in cortex tissue
E: Lion preantral folicle in the cortex tissue and F Maned wolf preantral follicle in cortex tissue. Credit: Jorge Luis Araújo Martins
In our bibliographic review, we found an article from Vitoria Keros which used a vitrification technique successfully in women, successfully preserving ovarian cortical tissues. This research was made with young women before they were submitted to oncologic treatment in order to preserve their fertility after the treatment. The article didn’t relate that ovary samples produced embryos and cubs after vitrification but was demonstrated that vitrified ovarian cortical tissue from women obtained better morphological results than the classic technique of slow freezing.
After a review, we did some pilots experiments, adapt this Keros technique for cats and drafted our experiments to try to achieve our goals. In one year of work, we performed three experiments that helped us to advance and validate an ovarian cortex vitrification technique for felines, which allowed us to find live preantral follicles in an ovary cortex, even after transport for up to 24 hours at 5°C following ovarian cortex tissue vitrification. The experiments were:
- Validation of Neutral Red as a biomarker for preantral follicles of carnivores;
- Vitrification of ovarian cortex of domestic cats (Felis catus) after 24-hour transport
- Comparison between slow freezing and vitrification of the ovarian cortex of a female lion (Panthera leo) followed by in vitro culture of a short period after vitrification.
This research has brought results and strategic information that will have a positive impact on the conservation strategies of wild carnivores.
In experiment 1, Neutral Red was validated in order to use this as a biomarker for preantral carnivorous follicles. Why is this good news? Because the vast majority of techniques that allow us to evaluate the viability of preantral follicles inserted in the ovarian cortex require the death of the tissue in order to say, after the analysis, whether this tissue would be alive or not. Through the use of morphological and molecular parameters, conclusions were drawn as to whether the primordial follicle cells were alive or dead. Neutral Red allows us to make this evaluation without cell death, and, thus, the set of primordial follicles present in the tissue sample can, after having its viability evaluated, be used for cryopreservation or other purposes.
The great advantage of this technique is that it will allow for serial analysis of the same set of follicles along the timeline. This technique opens up many possibilities for experimental designs involving primordial follicular research and the testing of in vitro protocols aimed at different goals. The protocol that we developed was validated for 5 carnivores species. This work received an Honorable Mention at the Latin American Congress on Animal Reproduction in 2015 in Santiago, Chile.
Experiment 2 was the heart of the research and was published in the journal Cryobiology in 2018. We use histological and ultrastructural analyses combined with Neutral Red to measure the cell’s (preantral follicle’s) viability. In this research, we have been able to demonstrate that it is possible to transport feline ovaries for up to 24 hours at 5 °C and to dissect and vitrify the ovarian cortex tissue using a protocol developed for humans. We also showed that after thawing this sample, it is possible to obtain a tissue with hundreds of viable primordial follicles. For the first time, it has been demonstrated that it is possible to apply this technology in a feline cortex using low-cost and highly adaptive techniques for field conditions. These results open the possibility of starting a genetic bank for wild feline females in an effort to minimize the great genetic erosion that is underway, storing the precious genetic material of the wild cat species to use in the future.
Finally, in experiment 3, we were able to apply the Neutral Red and ultrastructural analyses and the human-adapted vitrification protocol (developed in experiments 1 and 2) in 4 lion ovaries from Copenhagen Zoo. We added to this research the comparison of results between vitrification and slow freezing protocols. After thawing, we subjected these samples to a 7 day period of in vitro incubation. After one week, we reevaluated all the samples. The results were impressive. After 7 days in vitro, only the thawed samples from the vitrification protocol had hundreds of live primordial follicles, while the slow freezing samples had none. In this way, we have proven that vitrification, a portable and low-cost technique (compared to the slow freeze), is more efficient than slow freezing. If we scale this protocol for zoos and conservationists in general, it will allow worldwide amplification of this gene bank strategy with the collection of this precious genetic material from wild feline species that are threatened with extinction. These data are still unpublished.
All of this is very good news, but in fact, biotechnology will never replace the natural technology of species reproduction. Even biotechnology will make no difference if conservation strategies are not geared toward the preservation of populations in free-living and natural areas that are strategic to biodiversity, like the Amazon. Today, this kind of tool is important because of the borderline situation that we face, immersed in a mass extinction event. What is expected to be the real focus of conservation is a broad change in the way we relate with nature.
Respect it. Be grateful for it. We are part of the life chain and we couldn’t live alone on this planet. This consciousness has to be sown in schools, diffused in our habits of consumption, and guide the market and society toward a more solidarity economy — greener and low-carbon.
These findings are described in the article entitled Cat preantral follicle survival after prolonged cooled storage followed by vitrification, recently published in the journal Cryobiology. This work was conducted by Jorge Luis Araújo Martins, Maria Denise Lopes, Fabiana Ferreira de Souza, and Fabio Sossai Possebon from UNESP, and Gudrun Wibbelt and Katarina Jewgenow from the Leibniz-Institute for Zoo and Wildlife Research.
References:
- JEWGENOW, K.; WIEDEMANN, C.; BERTELSEN, M. F.; RINGLEB, J. Cryopreservation of mammalian ovaries and oocytes. International Zoo Yearbook, v.45, p.124-132, 2011.
- KEROS, V.; XELLA, S.; HULTENBY, K.; PETTERSSON, K.; SHEITKHIN, M.; VOLPE, A.; HREINSSON, J.; HOVATTA, O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod, v. 24, p.1670-83, 2009.
- LUVONI, G. C.; TESSARO, I.; APPARICIO, M.; RUGGERI, E.; LUCIANO, A. M.; MODINA,S. C. Effect of vitrification of feline ovarian cortex on follicular and oocyte quality and competence. Reprod Domest Anim, v.47, p.385-91, 2012.









