Glucose is the most important source of energy for living organisms. Living organisms rely on energy derived from glucose to run their biological processes. During cellular respiration, the body breaks down glucose and converts that chemical energy into the form of ATP. The majority of glucose used by heterotrophs is gained from external sources; i.e. eating food.
However, several organisms have developed metabolic pathways for creating glucose from non-carbohydrate compounds. Gluconeogenesis is the process of synthesizing glucose from non-organic carbon sources. When external sources of glucose are not readily available, the human body uses gluconeogenesis to keep create glucose and keep blood sugar levels sufficient.
In vertebrate mammals, the majority of gluconeogenesis takes place in the liver and to a lesser extent in the kidneys. Gluconeogenesis and glycolysis are two of the main mechanisms the body uses to regulate glucose levels. The two processes work together to keep glucose levels within a certain range.
In this article, we will take an in-depth view of gluconeogenesis and how the body uses gluconeogenesis to regulate blood sugar levels. First, let’s take look at the chemical structure of glucose.
What Is Glucose?
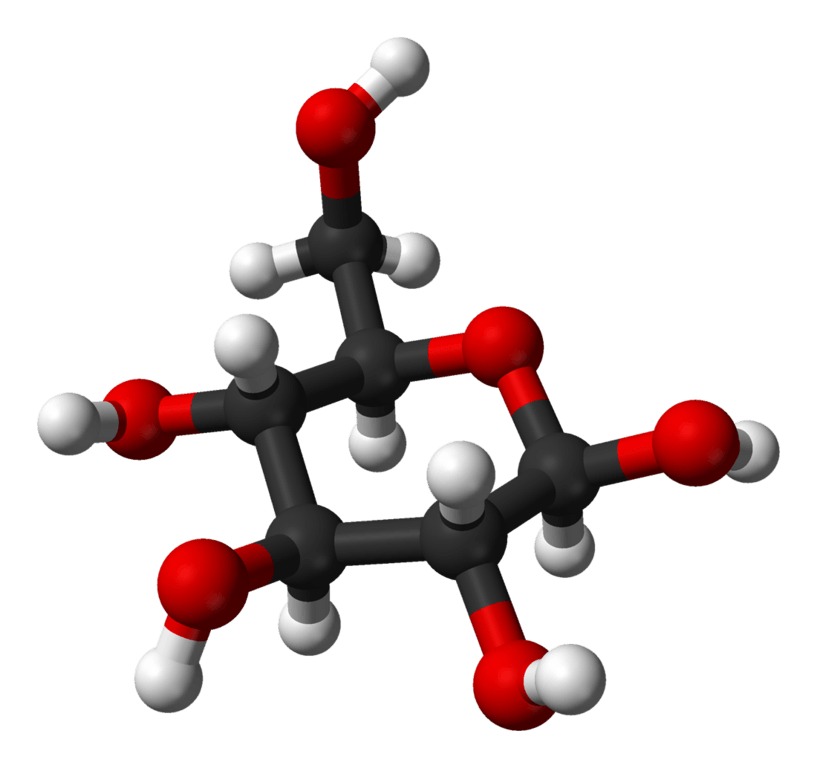
Glucose is a simple sugar (monosaccharide) with a chemical formula of C6H12O6. Glucose is the simplest and most abundant saccharide. A single molecule of glucose consists of a 6-carbon backbone bonded to 5 hydroxyls groups [OH] and a single aldehyde group [H(C=O)]. In solid form, glucose normally exists in a closed ring structure, but in aqueous solutions, it opens up into a linear chain structure.
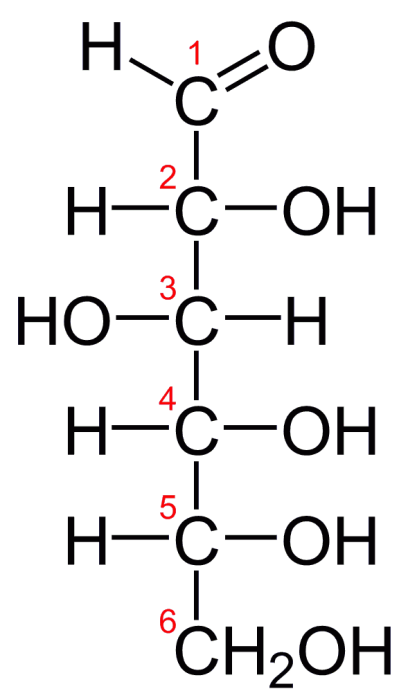
The linear structure of glucose. Credit: “D-glucose chain” via WikiCommons CC0 1.0
Glucose is useful for metabolic purposes because it has a lot of chemical energy wrapped up in its chemical bonds. The process of glycolysis breaks down glucose, releasing the energy stored in its bonds and converting it into the form of ATP. During cellular respiration, the 6-carbon glucose is broken down into two 3-carbon molecules called pyruvate, which are later oxidized.
Gluconeogenesis Overview
The overall chemical reaction for gluconeogenesis is:
2 pyruvate + 4ATP + 2GTP + 2NADH + 6H2O → Glucose + 4ADP + 2GDP + 6Pi +2NAD+ + 2H+ (ΔG = −38 kJ/mol)
Essentially, during gluconeogenesis, 2 molecules of pyruvate and 6 molecules of water are converted into glucose. 4 molecules of ATP and 2 molecules of GTP are dephosphorylated, resulting in the formation of 6 phosphorus units. Generally, the pyruvate used for gluconeogenesis is derived from lactate, glycogen, and amino acids.
In some ways, gluconeogenesis can be seen as the opposite of glycolysis. During glycolysis, glucose is broken down into pyruvate. During gluconeogenesis, pyruvate is used to synthesized glucose. The process of gluconeogenesis is not, however, simply a reversal of the reactions involved in glycolysis. Gluconeogenesis involves different reactions because the equilibrium of glycolysis heavily favors the pyruvate side of the reaction. As such, gluconeogenesis pathways in the body utilize enzymes not seen in glycolysis in order to move thermal equilibrium to the side of glucose production.
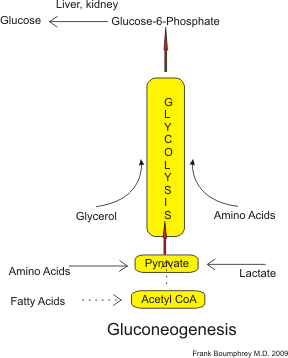
Simplified process of gluconeogenesis. Credit: Boumphreyfr via WikiCommons CC BY-SA 3.0
Glycolysis results in a decrease in the free energy of the chemical system which is why it produces energy. Most energy produced during glycolysis happens due to three steps that are irreversible under normal conditions. The steps are: the conversion of glucose to glucose 6-phosphate, the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, and the conversion of phosphoenolpyruvate into pyruvate. These 3 reactions are mediated by the enzymes hexokinase, phosphofructokinase, and pyruvate kinase, respectively. Gluconeogenesis pathways use different metabolic reactions to bypass these irreversible reactions of glycolysis.
Steps of Gluconeogenesis
Step 1: In the first step of gluconeogenesis, pyruvate is carboxylated to form oxaloacetate. This reaction uses up one molecule of ATP. The pyruvate is then phosphorylated to create phosphoenolpyruvate at the expense of one molecule of GTP. The formulae for these two reactions are:
pyruvate + CO2 + ATP + H2O ⇔ oxaloacetate + ADP + Pi + 2 H+
oxaloacetate + GTP ⇔ phosphoenolpyruvate + GDP + CO2
The two enzymes that catalyze the first and second reactions are called pyruvate carboxylase and phosphoenolpyruvate carboxykinase, respectively. Overall, these two reactions can be reduced to:
pyruvate + ATP + GTP + H2O ⇔ phosphoenolpyruvate + ADP + GDP + Pi + 2H+
The first reaction, the conversion of pyruvate into oxaloacetate, occurs mostly in the mitochondria of cells. Aftward, the oxaloacetate is then shuttled to the cytoplasm where it is phosphorylated into phosphoenolpyruvate.
Step 2: The next few reactions of gluconeogenesis are the same as in glycolysis, just the reverse. Phosphoenolpyruvate is metabolized to form fructose 1,6-bisphosphate.
Once fructose 1,6 phosphate is formed, it needs to be converted into fructose 6-phosphate. This reaction is as follows:
fructose 1,6-bisphosphate + H2O → fructose 6-phosphate + Pi
The main enzyme that facilitates this reaction is called fructose 1,6-bisphosphatase. This reaction is essentially a reversal of the reaction that forms 1,6-bisphosphate in glycolysis, though separate enzymes are involved so the reaction can have a net-positive energy gain.
Step 3: During the final step of gluconeogenesis, fructose 6-phosphate is readily converted into glucose 6-phosphate. Typically, gluconeogenesis ends here. Gluconeogenesis normally does not result in the formation of free glucose molecules because those can diffuse out of the cell easily. Instead, gluconeogenesis terminates with the formation of glucose 6-phosphate, which is incidentally the product formed during the very first step of glycolysis. The body regulates to the production of free glucose by restricting the enzyme responsible for this reaction to special tissues whose main duty is to maintain glucose homeostasis. These tissues are located mainly in the liver and kidneys.
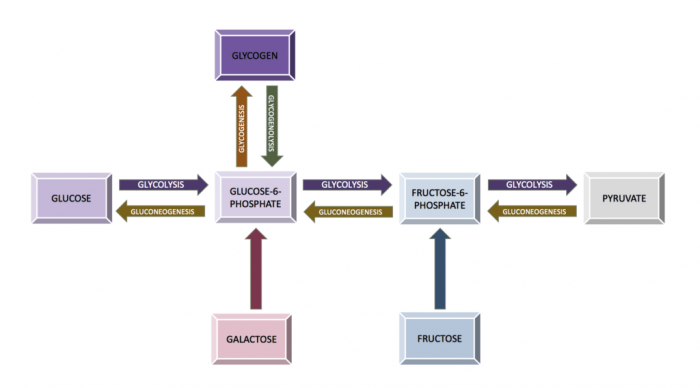
The relationship between the glycolysis pathway and the gluconeogenesis pathway. Credit: Eschopp via WikiCommons CC BY-SA 4.0
Differences Between Glycolysis and Gluconeogenesis
One might think that gluconeogenesis should just be the reversal of glycolysis. This cannot be for one major reason; glycolysis is an exogenic reaction meaning that it results if a loss of free energy (energy is produced). The reversal of this process is endogenic (requires energy to proceed).
The stoichiometry of gluconeogenesis is as follows:
2 pyruvate + 4ATP + 2GTP + 2NADH + 6H2O → Glucose + 4ADP + 2GDP + 6Pi +2NAD+ + 2H+ (ΔG = −38 kJ/mol)
In contrast, the stoichiometry for the reversal of glycolysis is:
2 pyruvate + 2 ATP + 2 NADH + H2O → glucose + 2 ADP + 2Pi + 2NAD+ (ΔG = 84 kJ/mol)
Notice that the enthalpy of change for the reversal of glycolysis is a positive value, meaning that the net energy gain is negative (the reaction takes up energy). Additionally, notice that the production of pyruvate from glucose in glycolysis produces 2 molecules of ATP. The synthesis of glucose from pyruvate during gluconeogenesis requires 4 ATP molecules and 2 GTP molecules. This means that gluconeogenesis requires the hydrolysis of 6 nucleotide triphosphate molecules. The extra cost associated with gluconeogenesis is 4 more nucleotide triphosphate molecules. The addition of the 4 triphosphate molecules per molecule of produced glucose turns a normally energetically unfavorable reaction (reversal of glycolysis, ΔG = 84 kJ/mol) into an energetically favorable reaction (gluconeogenesis ΔG = −38 kJ/mol).
How Is Gluconeogenesis Regulated?
Global gluconeogenesis is regulated by glucagon released when blood sugar levels are low. The release of glucagon stimulates the phosphorylation of enzymes involved in glycolysis, which slows the rate of glycolysis. Insulin is the counter to glucagon and stimulates glycolysis. This is why type II diabetics cannot control their blood sugar levels. Patients with type II diabetes are resistant to insulin and have excess levels of glucagon. As such, insulin cannot stimulate glycolysis and inhibit gluconeogenesis, leading to hyperglycemia.
Gluconeogenesis and glycolysis are two major pathway that regulates blood glucose levels. These two pathways do not operate independently though, for if they did, then the interplay of the two would result in a futile cycle: glycolysis would make pyruvate only to be reconstructed into glucose, only to be broken down into pyruvate, etc.
The two pathways can regulate each other because the enzymes involved in either reaction have a tendency to inhibit the mechanisms of the other pathway. These feedback mechanisms control the rate of each process. For example, in gluconeogenesis, the enzyme glucose 6-phosphatase that facilitates the conversion of glucose 6-phosphate into glucose is located mainly in the endoplasmic reticulum. In contrast, hexokinase, the enzyme that helps convert glucose to glucose-6 phosphate in glycolysis, operates mainly in the cytosol. This reaction is regulated by the cells capacity to transport glucose 6-phosphate to the endoplasmic reticulum.
As another example of mutually inhibiting mechanisms, In glycolysis, phosphofructokinase, the enzyme that helps turn fructose 6-phosphate into fructose 1,6-bisphosphate is inhibited by the presence of ATP. This makes sense because the end goal of glycolysis is to make ATP. Consequently, a buildup of dephosphorylated ADP or AMP stimulates the action of phosphofructokinase.
In contrast, fructose 1,6 bisphosphatase, the enzyme that helps turn fructose 1,6-bisphosphate into fructose 6-phosphate during gluconeogenesis, is stimulated by ATP and inhibited by ADP and AMP. This is the exact opposite behavior of phosphofructokinase, so the enzymes interact to make sure only one of them will be fully active at a given time.
To summarize, gluconeogenesis is a metabolic pathway that results in the production of glucose from non-carbohydrate substrates. During gluconeogenesis, pyruvate molecules derived from lactate, glycogen, and amino acids are converted into glucose. The steps of gluconeogenesis are very similar to the steps of glycolysis, just in reverse. Gluconeogenesis and glycolysis are two of the main metabolic pathways that regulate blood sugar levels. Gluconeogenesis and glycolysis can regulate each other because the enzymes involved in one pathway tend to inhibit the enzyme involved in the other pathway.









