
Organic luminescent solid materials have attracted a great deal of attention from the scientific community and the industry due to their benefits of a flexible structure and functional modification.
However, traditional organic luminescent materials show strong emission in dilute solution, but weak or even no emission in the aggregated state (nano-particle, micelles, solid films, and powder), which is the phenomenon of Aggregation-Caused Quenching (ACQ), the “Achilles’ heel” of organic luminescent materials. Researchers have made great efforts to use chemical, physical or industrial methods to avoid or minimize the ACQ effect, but they did not work well.
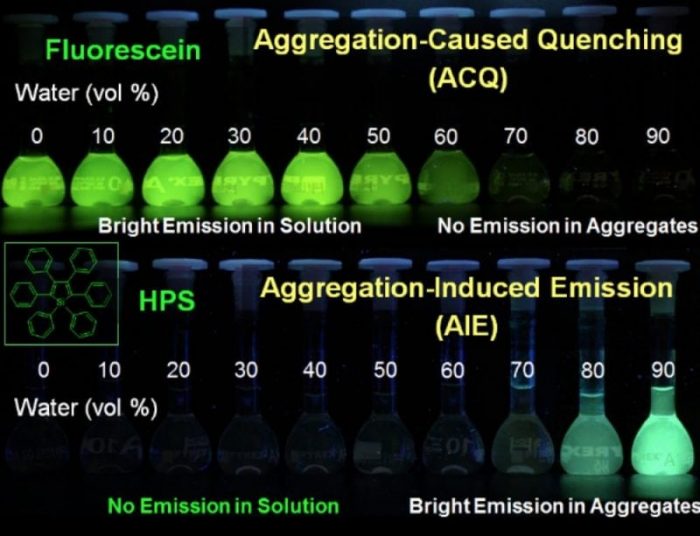
Fig.1 ACQ and AIE phenomenon (Reproduced with permission from Science China Chemistry)
The aggregation behavior of organic molecules in the solid state is an inevitable spontaneous process in terms of enthalpy and entropy, therefore deliberately inhibiting the molecular aggregation cannot fundamentally solve the ACQ problem.
In 2001, our group proposed a new concept of ” Aggregation-Induced Emission (AIE)” in the field of photophysics. This is based on the observed phenomenon that propeller-shaped luminogens (Silole) show a relatively high efficiency of fluorescent quantum yield in their aggregated states. AIE phenomenon offers a radically novel and astoundingly convincing solution for the ACQ problem of organic luminescent molecules. All the while they break the shackles of light-emitting efficiency and constraints of traditional thinking urging researchers to rethink the classical optical law and transition processes (Fig. 1).
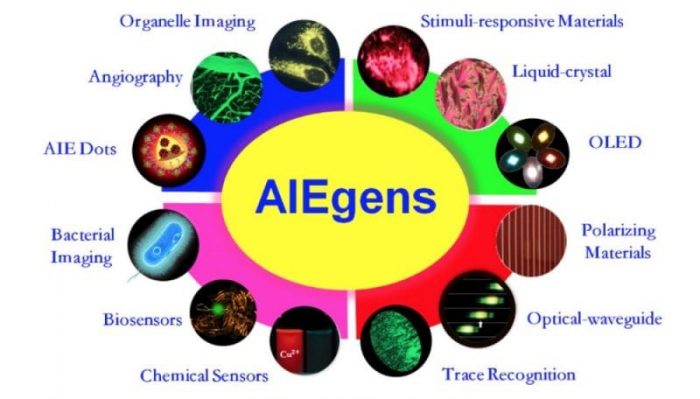
Fig.2 AIEgens’ applications (Reproduced with permission from Science China Chemistry)
Compared to the traditional organic fluorescent materials, the outstanding contributions of AIE is that it solves the problem of insufficient luminescence of organic materials in the aggregated state, which is the common form of luminescent materials in practical applications. For example, flexible display and lighting based on OLED is the breakthrough technology. While the preparation of organic thin films with highly efficient luminescence is the key point to the commercial market, the AIE materials applied in this area have displayed potential advantages.
After seventeen years of development, the AIE materials have expanded their applications in almost all fields such as:
- Responsive materials to stimuli (pH, temperature, solvent, pressure etc.)
- Reversible sensor intelligent materials
- Highly efficient OLED display and lighting materials
- Optical waveguide materials
- Selective chemical sensing materials
- Trace tracing materials and bioimaging materials used in biological systems such as organelles, virus, bacterial and blood vessels.
Among these, the application of AIE fluorescent probes in specific organelle bioimaging and long-term tracking is highly anticipated (Fig. 2).
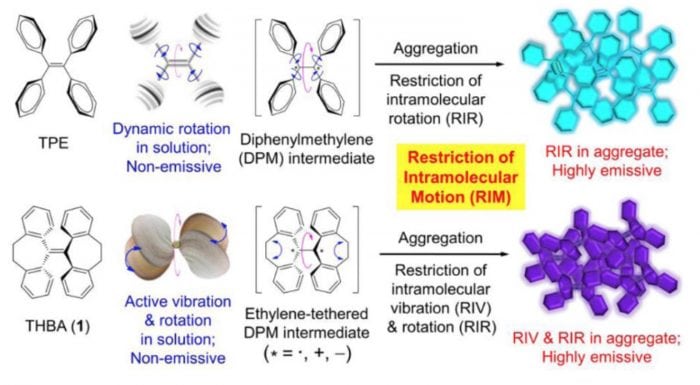
Fig.3 The luminescence mechanisms of AIEgens (Reproduced with permission from Science China Chemistry)
Developing AIE’s from concept to science lies in precise molecular design and functional modification of AIE molecules. The combination of experimental results and computational simulation indicates that the RIM (Restriction of Intramolecular Motion) theory is the working mechanism of AIE molecules, which includes RIR (Restriction of Intramolecular Rotation) theory proposed in 2003 and RIV (Restriction of Intramolecular Vibration) theory proposed in 2014. The RIM theory is popular and easy to understand, and thus brings instructive significance to the idea of AIE molecular design. The mechanism has greatly facilitated the development of AIE research field.
The quantity and number of citations of literature related to AIE have grown exponentially. There are more than 1500 institutes of scientists working in the AIE field in over 80 countries and regions around the world. In 2015, this topic of AIE ranked no. 2 in the areas of Chemistry and Materials Science by Thomson Reuters in its report on Research Fronts 2015. In 2018, our groups won the National Natural Science Award (China, 1st) for our original and leading work in AIE area.
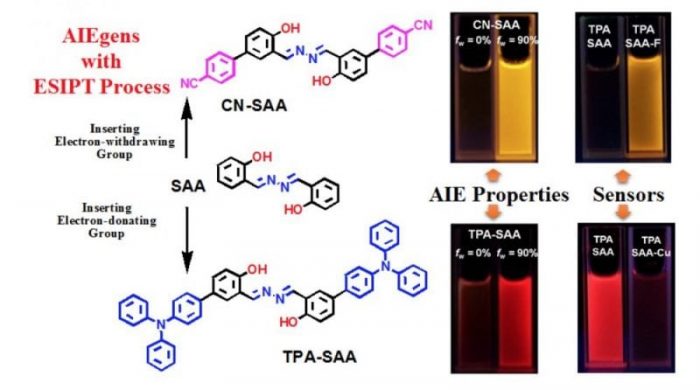
Fig.4 AIEgens example for their ion sensor characteristics based on SAA-structure (Reproduced with permission from Science China Chemistry)
Salicylaldehyde azine (SAA) is a typical molecule with excited-state intramolecular proton transfer (ESIPT) process, which consists of hydrazine and salicylaldehyde Schiff base, and its derivatives exhibited obvious AIE characteristics with highly distinguishable fluorescence performance and have been widely applied in chemosensors, bioprobes, and bioimaging etc. In fact, the active hydrogen of phenolic hydroxyl in SAA derivatives plays an important role in the ESIPT process. Both its form and transferability determine the fluorescence “turn-on/turn-off” and the quantum yield of the keto form in the aggregation process. However, the detail studies on the structure-properties relationship between the activity of hydrogen proton (or the acidity of the phenolic hydroxyl group) are relatively few.
Here, we constructed two SAA derivatives with different groups at the para-position of a phenolic hydroxyl group and investigated their effect on the ESIPT process of the generated luminogens (CN-SAA and TPA-SAA). By comparing the difference in NMR spectra, solvation effect, the absorption/fluorescence spectra in the aggregation process, and different pH buffers, we found that the substituents significantly impacted the excited state.
After inviting F– as a probe in MeCN solution, this activity difference became more obvious, and TPA-SAA exhibits great potential of quantitative F– analyzing. Meanwhile, its emissive nanoparticles in aqueous solution show better selectivity and sensitivity to Cu2+ than CN-SAA.
These findings are described in the article entitled Electronic effect on the optical properties and sensing ability of AIEgens with ESIPT process based on salicylaldehyde azine, published in the journal Science China Chemistry. This work was led by Zhiming Wang, Fan Zhou & Ben Zhong Tang from the South China University of Technology.









