Storage of spent nuclear fuel is a hot topic in many countries that are using or have used nuclear power in the past. Spent nuclear fuel is both chemically and radioactively toxic, and there is not a straightforward solution established as to how the fuel will be stored or treated.
The safety assessment of a final repository plays a key role in development and design, as well as in public debate and political decision making. Spreading of long-lived radionuclides could have adverse environmental and societal effects, which is why an accurate assessment is crucial.
Final nuclear waste repositories are designed to last for 1 million years because of the long half-lives of the actinides present in nuclear fuel, such as uranium, plutonium, and neptunium. Because of the extensive time scales that are relevant, accurately assessing the chemistry at the canister interface and the longtime structural integrity of the canister is difficult.
Perhaps the first experiment on radionuclide migration from nuclear fuel was performed roughly two billion years ago in Oklo, Gabon. Here, there existed several sites with natural self-sustaining fission due to the uranium-rich minerals which reached criticality in contact with groundwater as a moderator. The fission reactions were sustained during brief periods of time, after which the water was evaporated due to the fission heat. As the mineral deposits were gradually filled with water, the process started again. The long-lived radionuclides formed in the process have been remarkably well retained in the bedrock, which has given a strong indication that crystalline rock is well-suited for the prevention of radionuclide migration.
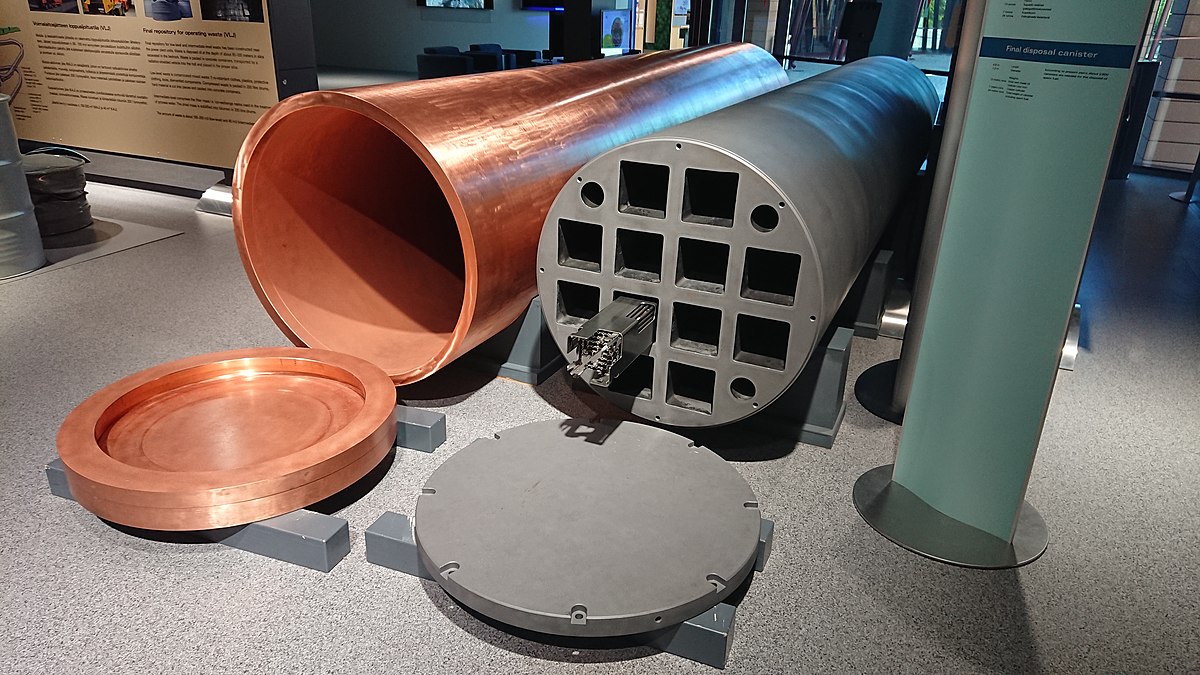
In the Swedish and Finnish final repository model (KBS-3), the canisters are put several hundred meters into the ground, surrounded by granite bedrock. The design also involves a bentonite clay surrounding the canister, which expands in contact with water and further ensures the initial reducing conditions in the event of water intrusion.
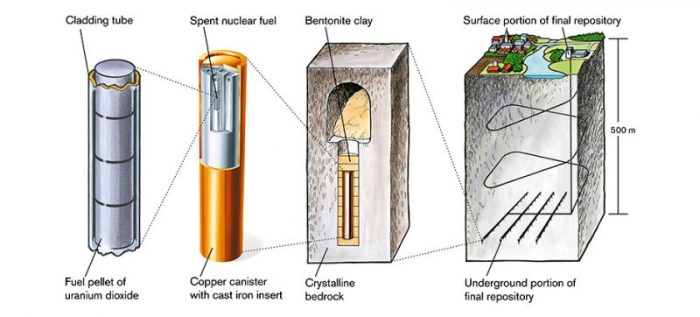
Figure 1 – The Swedish and Finnish final repository model. Courtesy of Swedish Nuclear Fuel and Waste Management Co, Illustrator: Jan Rojmar
Canister and Nuclear Fuel Chemistry
In the event of a canister break due to stress corrosion cracking, seismic activity causing shear stress on the canister, or other causes, groundwater intrudes into the canister, causing an instantaneous release of radionuclides which have accumulated in the Zircalloy cladding space. In contact with water, hydrogen is formed through the anoxic corrosion of the massive iron inserts, which can be seen in Fig. 1. according to Eq. 1 (as well as through radiolysis to a lesser extent):
(1)
In the nuclear reactor, fuel consisting of enriched uranium is fissioned to produce thermal power. In the process, a vast number of elements are formed from almost the entire periodic table. In order to simulate used fuel, SIMFUEL consists of polycrystalline uranium containing additions of Sr, Y, Zr, Mo, Ru, Rh, Pd, Ba, La, Ce, and Nd analogous to their proportions in used nuclear fuel at a specific burn-up (i.e. having been used to produce a specific amount of energy). The elements Mo, Ru, Rh, and Pd form a metallic alloy phase which can act as a chemical catalyst.
In the water intrusion scenario, the initially reducing conditions are strongly affected by the interaction between the radiation and the water. The β- and γ-emitters will constitute a major part of the radiation field during the first hundred years, after which α-radiation will dominate. The radiation breaks the bonds of the water molecules in the decay particle track, creating several thousand radical and molecular species per α-particle. Because of the low chemical reactivity of H2, the net redox conditions under α-radiolysis are oxidizing due to the high radiolytic yield of hydrogen peroxide, H2O2. The radiolysis products, therefore, oxidize the UO2 matrix, turning the highly insoluble U4+ form into the much more soluble U6+ form.
The protective hydrogen effect
There is, however, a mechanism that counteracts the oxidative dissolution in the water intrusion scenario. The hydrogen is believed to be catalytically activated on the metal particles, making it possible for the hydrogen to consume oxidants before they corrode the fuel surface. The mechanism of how the hydrogen effect works to protect the nuclear fuel is not yet proven; however, the main hypothesis is through a reaction between H· and OH·, which is a very strong oxidant formed as H2O2 dissociates on the fuel surface, to form water.
In this study, we have studied this mechanism through the use of a heavier hydrogen isotope, namely deuterium. Deuterium makes it possible to use laser spectroscopy isotopic analysis to study the heavier water HDO and D2O, formed through isotopic exchange and chemical reactions. The results show that the hypothesized mechanism seems highly likely, as significant amounts of HDO is produced, somewhat less than the consumed OH·. A slightly smaller fraction of the OH-radicals reacts further with H2O2, leading to decomposition into O2 and H2O. SIMFUEL is also shown to be relatively chemically inert, showing that the metallic inclusions also provide a stability against oxidation from H2O2 even without the hydrogen effect. As such, only a minor part, 0.02%, of the total H2O2 consumed in the reference case without dissolved hydrogen caused oxidative dissolution of the SIMFUEL.
The results indicate that hydrogen activation on metal particles will play a crucial role in the nuclear repository safety in the water intrusion scenario. The oxidation will be significantly suppressed due to this process, which will keep the nuclear fuel from dissolving over the required time spans.
These results are discussed in depth in the article The fate of hydroxyl radicals produced during H2O2 decomposition on a SIMFUEL surface in the presence of dissolved hydrogen, recently published in the Journal of Nuclear Materials. This work was conducted by Lovisa Bauhn, Niklas Hansson, and Christian Ekberg from Chalmers University of Technology, Patrik Fors from Vattenfall AB, and Kastriot Spahiu from Chalmers University of Technology and the Swedish Nuclear Fuel and Waste Management Co.









