
The production of domesticated shrimp broodstock is an expensive process which also requires time. However, the techniques currently used in most reproduction laboratories are not based on principles of animal ethics and, consequently, reduce broodstock lifetime. For example, the main technique used for inducing the female gonadal maturation is the unilateral eyestalk ablation, which results in the broodstock death after 4-5 consecutive spawns, due to the reproductive exhaustion.
Concerning the males, the main issue associated with their reproductive quality in captivity is the spermatophore melanization. This process is the major immunoeffector responses of crustacean which comprises cascade chemical reactions whose final product is the synthesis and deposition of melanin (Fig. 1). It has been demonstrated that these chemical reactions may be triggered by pathogens, urea, calcium ions, heat, and others.
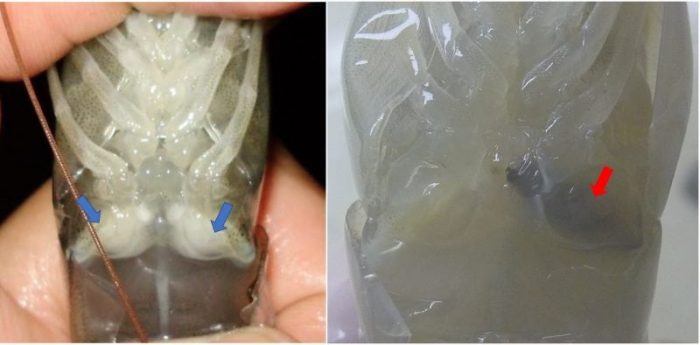
Figure 1: Shrimp non-melanized (blue arrows) and melanized (red arrow) spermatophores. (Credit: André Braga)
The synthesis of the spermatozoa and their packaging into spermatophores are observed in subadult males when they are around 6-8 g. From this phase, the shrimp synthesize sperm cells via a continuous process, which reaches its maximum from 17 g. Therefore, it is not necessary to induce males to maturation and, thus, they usually last longer than the females under captive conditions. Despite this fact, management of broodstock may cause the progressive reduction of the male reproductive quality.
In maturation laboratories, the natural copulation is targeted in order to obtain better larval production in terms of quality and quantity. However, a percentage of mature females are not successfully inseminated by the males, which depends on the maintenance of the environmental factors within the range recommended for shrimp reproduction. As a strategy to avoid losing the spawning of these females, some laboratories keep all-male tanks, aiming to collect spermatophores to be used for artificial insemination.
The artificial insemination in shrimp consists of externally adhering the sperm mass to the female thelycum (in case of open thelycum species) or internally inserting the spermatophore to the thelycum (in case of closed thelycum species) (Fig. 2). In both cases, the spermatophore is first collected from the male terminal ampoule. The manual method for spermatophore extrusion is widely used for this purpose and consists of compressing the coxae of the fifth pereiopod pair.
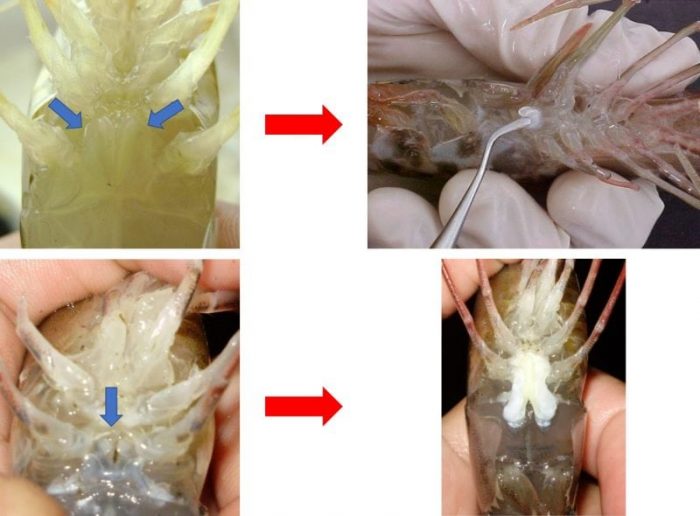
Figure 2: The pictures on the top show a spermatophore being inserted in a closed thelycum (blue arrows) female, whereas those on the bottom show the sperm mass externally adhered to the open thelycum (red arrows). (Credit: André Braga)
The association between manual extrusion and spermatophore melanization has been previously suggested in literature. However, no investigations proving this association or showing what happens after the manual extrusion at tissue level were available. We conducted an experimental study and were able to demonstrate for the first time that the manual extrusion method may cause spermatophore melanization.
In this 30-day study, pink shrimp males had their spermatophores manually extruded every second week, and sperm quality and histological analyses were conducted. We showed that survival decreased after the extrusion events, and the percentage of melanized spermatophore increased. The histological microscopy showed that an inflammatory process characterized by a heavy hemocytes infiltration into the spermatophore tissues was identified as the trigger to the melanization (Fig. 3).
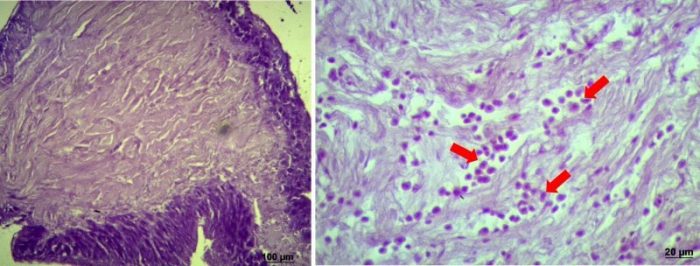
Figure 3: Histological microscopy of non-melanized (on the left) and melanized (on the right) spermatophores. Arrows indicate the hemocytic infiltration. (Credit: André Braga)
In addition, we demonstrated a progressive reduction of the sperm quality of manually-extruded males. This reduction is most likely a final phase of the process of hemocytic melanization of the spermatophore caused by the manual method. Figure 4 shows that the loss of the sperm quality was characterized by the reduction of the number of total and abnormal spermatozoa contained in the analyzed spermatophores.
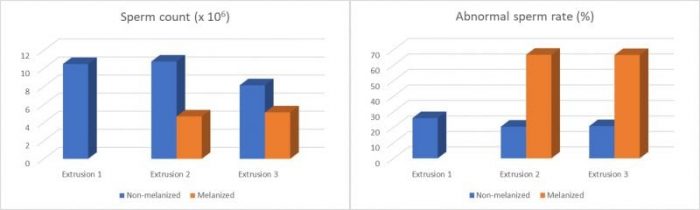
Figure 4: Sperm count and abnormal sperm rate of spermatophores analyzed during the experimental period. (Credit: André Braga)
Our findings comprise one more example that demonstrates the reduction of the reproductive quality associated with the methods currently applied in maturation laboratories. Therefore, they highlight the need for more ethical and efficient methods in aquaculture. Efforts in this direction also would represent positive cost impacts for commercial hatcheries.
These findings are described in the article entitled Hemocytic melanization in shrimp spermatophores, recently published in the journal Aquaculture. This work was conducted by André Braga, Vitalina Magalhães, Marta Klosterhoff, Luis Romano, Luís Poersch, and Wilson Wasielesky from the Federal University of Rio Grande, and Diogo Lopes from the University of the Santa Catarina State.









