
When you hear the word “fungus,” you might imagine the forgotten strawberry or piece of bread left in your refrigerator when you’re headed off to vacation. But the fungal kingdom represents much more than the fuzz on your bread. In fact, fungi are the most diverse group of eukaryotic organisms. They have a variety of ecological roles. Some fungi make our lives easier, while others can make us or our crops sick.
Recently, it was discovered that fungi and humans shared a common ancestor about 1.538 billion years ago, while plants and fungi shared an ancestor 1.547 billion years ago, making humans and fungi more closely related than fungi and plants. Fungal cells are similar to human cells in that they have nuclei and other organelles to process energy, but lack chlorophyll or chloroplasts (the photosynthetic structures) as found in plants. As many as thirty percent of genes associated with human diseases have similar copies in fungi making it possible to understand important human genes using experimental fungal organisms. In fact, a major project replaced almost 500 individual fungal genes with their human equivalents and nearly half of the replacements resulted in the yeast being “just fine”.
Several fungal species have been used as experimental organisms for decades. These fungi feature a short life cycle and a simple genome which researchers can compare to other eukaryotic groups, such as humans. Neurospora crassa, a fungus harnessed from an outbreak in French bakeries, was first used in genetics research in 1941 to detail the relationship between genes and enzymes. Two researchers, George Beadle and Edward Tatum, used x-rays to mutate spores of the fungus and track enzymatic activity. Another fungus called Saccharomyces cerevisiae, or baker’s yeast, has also been used domestically in doughs for over 4,000 years and plays a huge role in research on metabolism, aging, neurodegenerative diseases, and much more. This was the fungus used in the above-mentioned experiment where researchers replaced yeast genes with human genes. Both of these fungi remain popular research organisms today and a Google Scholar search of “Saccharomyces cerevisiae” resulted in nearly 17,000 results published in 2018 alone (September 6, 2018).
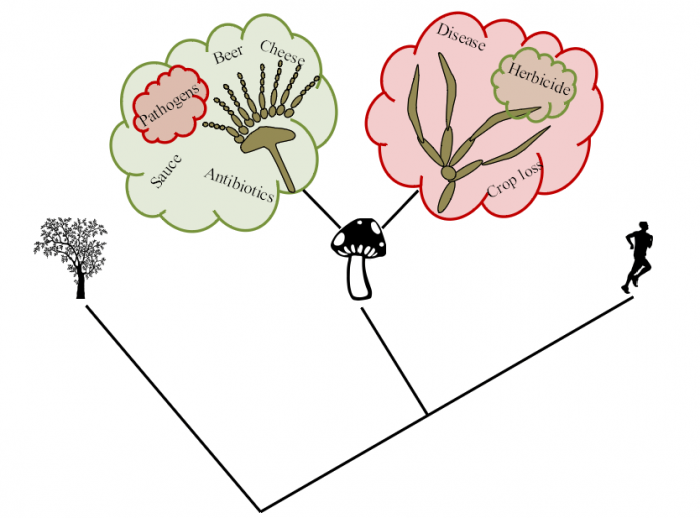
Figure 1. The different roles of Fungi: Fungi represents a dynamic class of organisms which are more closely related to humans on evolutionary scale as compared to plants. This group of organisms show varied roles and have both positive and negative roles. On the positive side, fungi have the beneficial role in making Beer, cheese, antibiotic and cooking sauce while some members of the same group act as pathogens. On the negative side there are many fungal species that cause plant diseases and pose a high threat by crop loss, however some members of this fungal family also act as biocontrol agents in preventing the pathogenic members. Figure courtesy Genetics Society of America
Using fungi as an experimental organism for research, we have domesticated fungi for our benefit. Some species, like Fusarium nygami, are used as a bioherbicide for weed control in African agriculture. Ancient Egyptians unknowingly utilized yeast in bread-making, beginning around 3000 B.C. The invention of the microscope in 1676 paved the way for Louis Pasteur to identify the biochemical processes behind yeast’s action in 1859. Today, new yeast strains are still being explored for bread, beers, and cheeses to develop yeast that make these products taste better, have lower production costs, and other desirable traits.
Despite over 1,500 yeast strains existing in the wild, only very few are used in industry. This presents an opportunity to diversify those currently in use or to find species better suited for existing industrial roles. Using what we already know about yeast-insect ecology, some researchers have begun letting insects sniff out new, potentially useful yeasts. Specifically, researchers believe that insects may be attracted to ester-producing yeasts. Esters are molecules that can smell fruity and aromatic. Already, some researchers have used paper wasps to isolate a yeast species and brew a new style of beer. Finding these yeasts might make your next beer a bit tastier.
Like the Egyptians accidentally discovering that yeast leads to leavened bread, cheese was likely an accidental discovery as well, and shrouded in folklore. Cheese aged in the caves near Roquefort developed blue-green veins throughout the cheese that enhanced the flavor. The fungus Penicillium roqueforti turned out to be the primary ingredient for this transformation. Despite efforts to keep this particular fungus a secret, word got out and now Gorgonzola, Stilton, and other popular cheeses all use this species in the aging process. Penicillium camemberti, a close relative, is used for Camembert and Brie. Another fungal species, Aspergillus oryzae, is used to make soy sauce and miso, while Rhizopus oligosporus is used to make tempeh. These are just a few examples of the diverse range of food products that are possible due to fungi.
Beyond food products, fungi have given us so-called “miracle drugs” that are still in use today. The antibiotic penicillin was a recent, world-changing discovery thanks to fungi and bit of luck. Others made observations about the antibacterial properties of certain fungi, but it was Alexander Fleming who first saw the potential for Penicillium notatum and its antibacterial properties. After initial observations that P. notatum inhibited bacterial growth, he struggled for years to reliably produce large quantities of the active ingredient. It took the interest of Howard Florey and his large lab to finally test penicillin in animals and eventually humans. After their successes in treating infection, it remained difficult to produce sufficient quantities for large-scale medical use. With funding from the Rockefeller Foundation, a wild strain of Penicillium chrysogeum was identified that could produce 200x as much penicillin, and after mutating this new species using X-rays and UV rays, a strain was identified that produced nearly 1000x as much as Fleming’s original strain. The impact of mass-produced penicillin was immediate, as previously common causes of death could be treated easily. Millions of people have been saved due to penicillin and it is estimated that twelve to fifteen percent of Allied troops were treated and saved by penicillin during World War II.
Unfortunately, not all fungi are beneficial. Some fungal species have evolved to be pathogenic, infecting plant and animal hosts, and cause serious diseases. There are about 300 fungal species that are known to make people sick. Many fungal diseases in humans are more of an annoyance, but they can become life threatening, especially for those with weakened immune systems. For example, a “yeast infection” is often caused by the species Candida albicans. Infections such as ringworm, caused by the fungus Trichophyton, spread when people are in crowded conditions and became a problem during World War II for soldiers stationed in the South Pacific.
Fungal plant diseases affect the productivity of crops that feed the world. Despite intensive agrochemical input, diseases caused by fungi continue to destroy between 10%-23% of agricultural production worldwide each year. One of the most devastating examples of a fungus-like organism in agriculture is the potato blight (Phytophthora infestans), famous for causing the Great Irish Famine in the 1840s. Fungi that infect plants can also adjust to different environmental conditions, making it difficult to predict and combat disease. One recent example of a fungal pathogen threatening crop production is the wheat blast fungus (Pyricularia oryzae), which evolved in Latin America and has caused devastating wheat loss in Bangladesh during the last 2 years.
Some fungi can be both beneficial and pathogenic. The genus Fusarium, previously mentioned as beneficial, also includes a number of pathogenic species that infect crops. The most notorious among these is Fusarium oxysporum, a soil-borne plant pathogen that causes vascular wilt disease in several field and greenhouse crops, including tomato, potato, and melon. During recent years, an evolved strain of this fungus called Tropical Race 4 (TR4) has caused serious economic losses worldwide in banana plantations, hampering Asian agriculture and impacting banana exports. Fusarium wilt of banana (Panama disease) causes subtle symptoms, which often are initially undetected and may cause plant death in severe cases. Banana is a clonal crop and only one variety, Cavendish, is consumed worldwide. Current solutions remain unknown but a strain with a gene taken from a wild banana has shown promise in resistance to Fusarium wilt.
Fungi also need further attention, as recent research has demonstrated two alarming trends. First, farmers can treat their crops to help control fungi, but some fungi remain in ground reservoirs after harvest and those that can infect humans have increased resistance to treatments if people get sick. Second, filamentous fungi are gaining the ability to grow at previously unsuitable higher temperatures (termed “thermogenesis”). Although this ability could aid in developing novel applications of fungal biocatalysts for industry, it also poses a serious challenge if pathogenic species become suited to wider temperature ranges.
Fungi are both some of our greatest friends and fiercest foes. Understanding the relationships of fungi will provide novel resources that may lead to the discovery of new products and resources. It is important to utilize this underappreciated group in molecular and genetic research to help us harness and understand the benefits and dangers associated with fungi in our natural world. Further study into this kingdom is, indeed, required and more surprises and insights are certainly in store.









