Morphogenesis is the process through which organs and organisms acquire their shape during development. These changes in shape are the result of coordinated cellular movements that are integrated at the tissue level and are eventually governed by gene expression. To understand this process, our group took advantage of the fruit fly, Drosophila melanogaster, as a model to study in depth how morphogenesis is genetically regulated.
The set of instructions that direct animal development is encoded in its genome. Therefore, to ensure the correct form, size, and function of the organisms, this information must be translated into patterning mechanisms that dictate cellular behaviors such as proliferation, differentiation, and cellular and tissue movements. Although both processes — pattern formation (acquisition of cellular identities) and morphogenesis (acquisition of form) — have been studied in depth separately, much less is known about how specific genetic networks control specific cell dynamics. Using leg formation as our model, we set out to unveil how a three-dimensional structure is formed from a flat epithelium.
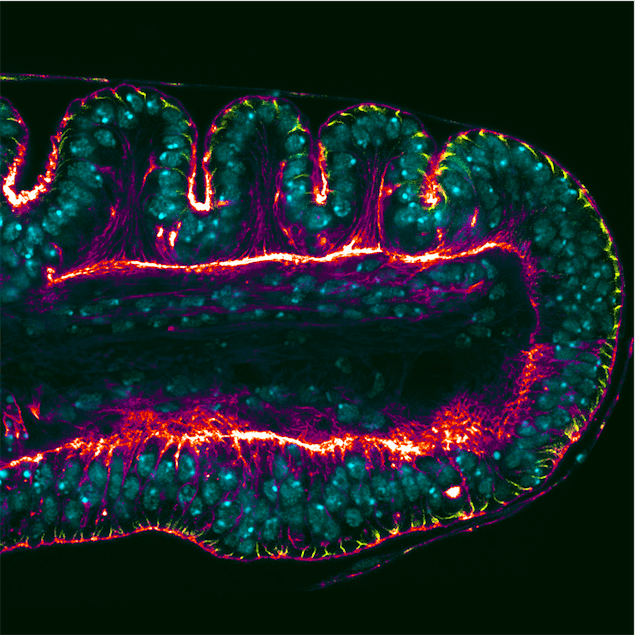
Drosophila adult cuticular structures (such as legs, wings, eyes…) develop as “imaginal discs” — epithelial sac-like structures that can be found inside the larva and that will form their corresponding adult parts upon metamorphosis. The leg imaginal disc is genetically subdivided into different domains that will form the future segments of the leg. A consequence of this territorial subdivision is the formation of the leg joints that allow appendage articulation. The joints are first prefigured during leg imaginal disc development as deep epithelial folds formed by coordinated cell shape changes through a process known as apical constriction.
An intuitive approach to understand epithelial morphogenesis is to compare it with origami, the art of creating tridimensional figures out of a sheet of paper. While in origami a set of instructions dictate how to fold the paper to obtain the final figure, during development, genetic information acts as the instructions that direct the cellular processes that change the shape of the epithelium.
Our choice of the leg imaginal disc as a model is not casual, as there is plenty of knowledge available about its genetic patterning; the genetic network that gives identity to the different segments of the leg is known to a great extent of detail. Back in 2014, our lab published a study in PLoS Genetics where we identified the gene dysfusion (dysf), which encodes for a transcription factor absolutely required for the formation of leg joints. We found that dysf acts by regulating the expression of a set of target genes that in turn control joint formation.
In our more recent work, also published in PLoS Genetics, we went a step further and explored the mechanisms responsible for epithelial fold and adult joint formation that are ultimately regulated by dysf. By comparing normal individuals with dysf mutants that lack epithelial folds and adult joints, we have been able to identify the molecular mechanisms that drive cell shape changes in this developmental context. Our study concludes that apical constriction is mediated by the GTPase protein Rho1, a protein that is widely known as a regulator of cell shape changes in epithelial sheets. Therefore, in the leg Rho1 activity is controlled by Dysf to form the characteristic epithelial folds in the leg imaginal discs.
Nevertheless, aside from solving the particular question about how Drosophila’s leg joints are formed, the true relevance of this work relies on understanding how the processes of pattern formation and morphogenesis are coordinated during development. We have been able to track joint formation from the genetic patterning of the leg to the cellular behaviors that sculpt the shape of the epithelium, and have identified Dysf as a key element to connect both processes.
These findings are described in the article entitled The transcription factor Dysfusion promotes fold and joint morphogenesis through regulation of Rho1, recently published in the journal PLOS Genetics. This work was conducted by Sergio Córdoba and Carlos Estella from the Universidad Autónoma de Madrid.









