
Mitochondria are powerhouse organelles in the cell. They are mainly responsible for generating ATP, a high-energy molecule used in various cellular activities. The ATP production occurs in the respiration process occurred inside its tiny compartments.
However, most proteins that build the mitochondria and required for its activity are not synthesized within, but rather by the protein-synthesizing machinery called ribosomes located in the cytoplasm, just as the majority of cell proteins. The question is, how do proteins, which are required to build mitochondria enter the organelle to do their job? The answer lies in the specialized protein import mechanism developed by mitochondria.
Mitochondria are thought to have arisen from the incorporation of oxygen-consuming prokaryotes by ancestral eukaryotic cells billions of years ago. This evolution, called the endosymbiotic process, is a major turning point that gave rise to modern eukaryotes such as fungi, plants, and animals. Plants, however, have a unique feature compared to others as they have not only one but two endosymbiotically-derived organelles, mitochondria, and plastids. Both happen to be mainly responsible for generating energy and molecules required for living. Interestingly enough, some proteins synthesized in the cytoplasm may be dual targeted to both mitochondria and plastids. As a result, some of the mitochondrial components in plants are exclusive and have become of considerable interest among plant molecular biologists to elucidate.
Despite its tiny size, the protein-targeting mechanism in the mitochondrion is surprisingly complex and involves various protein complexes. It’s extremely busy all the time! The mitochondrion is a double membrane-bound organelle. Consequently, there are also two major components of protein translocases reside in the outer and the inner membrane, to move proteins across subcompartment from the outer membrane to the inner membrane, the inner membrane space (IMS) between them, and the matrix.
In the outer membrane, there is the translocase of the outer membrane (TOM) and the sorting and assembly machinery (SAM) complexes responsible for initially recognizing targeted proteins and other macromolecules. Whereas in the inner membrane, the translocase of the inner membrane (TIM) complex serves as the protein selector. In the IMS, protein sorting generally performed by the mitochondrial inner membrane space import and assembly (MIA) complex. Plant mitochondrial import components can be seen in the figure. Together, these translocase components make up four major pathways in translocating targeted proteins with different features. These are the general import pathway, the carrier import pathway, the MIA pathway, and the SAM pathway that mainly responsible for transporting proteins with pre-sequence, proteins with internal signals, twin-cysteine proteins, and β-barrel proteins, respectively.
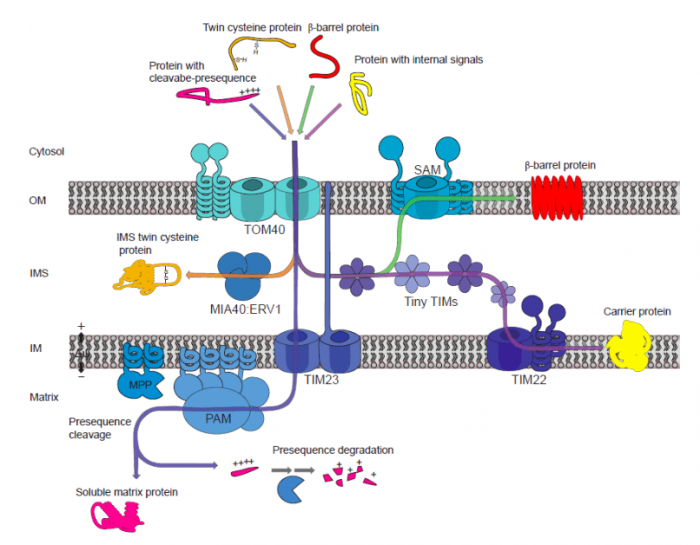
Figure 1. Major translocases in plant mitochondria. Republished by the author of 10.1042/BCJ20170521.
These complexes have multiple protein parts which act together in identifying the signals and transport the proteins to different organelle locations. Interestingly, even though the translocase complexes have been observed in all eukaryotes, the components and structural arrangement of import components in plants can significantly differ from the other. For example, some TOM constituents were not found in plants but have been characterized in yeast (Saccharomyces cerevisiae) such as Tom70 and Tom22/9.
On the other hand, some components are exclusive to plants such as Om64, which is responsible for the transport of, among other things, a subunit of ATP synthase. ATP synthase is an enzymatic complex that is responsible for producing ATP, the main role of mitochondria. The deletion of Om64 along with other essential TOM components is lethal to plants, demonstrating their essential role in mitochondrial biogenesis and activity.
A recent systematic proteomics study of Arabidopsis mitochondrial import components demonstrated that not all components have the same turnover rate. Some have a relatively faster turnover, meaning that their change in abundance is higher than other important components. These “fast” import components include Tom20 and Tim17. It is predicted that these “fast” components are needed for growth and developmental stages of plants which requires fast protein synthesis. It is also worth to mention that Tim17 homologues are indeed pivotal in plant germination and stress response.
These complicated arrangements of protein import raise the next question: how do these complexes recognize which proteins may enter and which one should go to a specific location? The key to this import lies in the targeting signal within the protein sequence.
Targeting peptides are a set of amino acid sequences within targeted proteins that correspond with the recognition and sorting by the protein import components. There are mainly two types of targeting signals: cleavable and non-cleavable. The majority (around 70%) of mitochondria-destined proteins contain cleavable targeting peptide, called presequences, which will be cleaved upon import and further digested into amino acid through a multi-step processing pathway. Recent studies have shown that the secondary structure of targeting peptide determines the recognition by import components rather than its sequence. For example, mitochondrial presequences often form amphiphilic α-helices that are recognised by TOM complex. This may explain why some proteins are targeted to multiple organelles which is due to the preference of translocases to recognise a structural feature rather than exact sequence.
Plant mitochondrial protein import components have low conservation compared to other eukaryotes. Distinct protein transport components have been characterized to date which structurally and regulatorily differ from closely-related proteins of other eukaryotes. Thus, it is difficult to characterize new import components solely from sequence analysis and phylogenetics studies. Genetics and biochemical studies may shed light in characterising these new import components that act as the gatekeeper of mitochondrial biogenesis and activity as well as growth and stress-responsive regulations in plants.
These findings are described in the article entitled Plant mitochondrial protein import components: the ins and outs, recently published in Biochemical Journal. This work was conducted by Abi S. Ghifari, Mabel LA. Gill-Hille, and Monika W. Murcha from The University of Western Australia.









