
Since Alexander Fleming discovered penicillin in 1928, millions of human lives have been saved thanks to the use of antibiotics. However, with their extensive application, antimicrobial resistance arises, which limits the effect of conventional therapy.
The design of slow-release delivery systems is one of the attempts to decrease the induction of antibiotic resistance. Besides “smoothing out” the intake of the medication, one of the ideas behind slow release is to avoid the sudden stop of the treatment: many patients tend to interrupt the antibiotic intake once they feel better, and thus the incomplete treatment may aggravate the development of antibiotic resistance. With slow release systems, patients have to take their pills less frequently, so they are more likely to complete the treatment.
Hosting the drug in a “matrix” is a typical strategy to achieve the slow release properties. Cellulosic polymers (such as hydroxypropylmethylcellulose) are very frequently used as matrices — most of them are artificially synthesized.
The group of A. Rivera at the Zeolite Engineering Laboratory (IMRE, University of Havana) has spent several years exploring the use of porous materials as hosts for the slow release of drugs — including antibiotics. Among them, zeolites and clays have concentrated most attention. One advantage of these matrices is the fact that they can be easily found in natural forms (typical mud is basically made of clays!) which has been demonstrated to be innocuous for the human body.
However, in order to understand in detail the mechanisms of the incorporation and release of drugs in and from these matrices, it is important to investigate first their “pristine” versions. This is exactly what Ph.D. student D. Hernández and coworkers at Rivera’s group — in collaboration with colleagues from France and Poland — have reported in their paper “Synthetic clay mineral as nanocarrier of sulfamethoxazole and trimethoprim” published in Applied Clay Science earlier this year.
They have succeeded in incorporating into the clay Li-Fluorohectorite a couple of very popular antibiotics (sulfamethoxazole and trimethoprim), widely used to treat bacterial infections spanning from the middle ear to the intestine. The systematic application of spectroscopic, structural and thermal techniques of analysis demonstrated that the drugs are effectively hosted in the clay. Each drug, however, finds its own way of hosting: while the sulfamethoxazole is adsorbed at the surface of the matrix grains, the trimethoprim is intercalated — i.e., it is “sandwiched” between the structural layers of the clay, see figure.
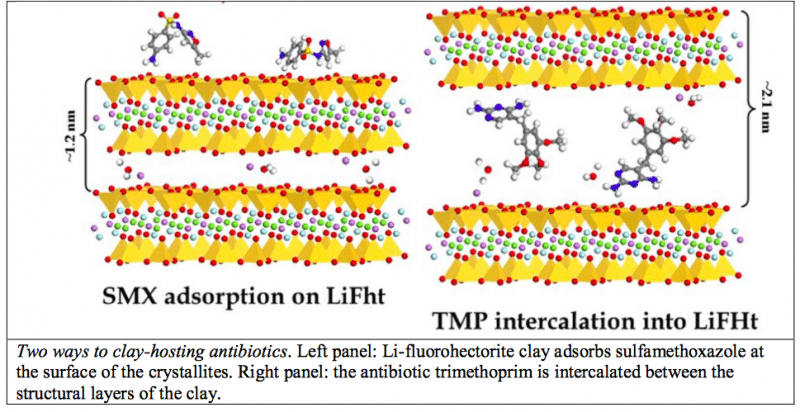
Credit: A. Rivera
The authors also study how the drugs are released from the clay host in an in vitro environment that mimics the chemical conditions of the gastrointestinal tract. The results show that both antibiotics take nearly 8 hours to be released from the matrix, which indicates an excellent potential for slow-release medical applications. Moreover, the fact that the release details depend on the pH of the environment, clay-based slow release systems may be designed to target, for example, different sections of the gastrointestinal tract.
So, when animals and eventually humans practice geophagy — an academic way to describe dirt eating —it cannot be taken as a simple desire to fill up an empty stomach. Clays and zeolites contained in the ground may adsorb toxic substances from the stomach, and also may provide micro-nutrients such as iron and zinc. But, in the light of the work of Rivera’s team, “dirt” can be also transformed into an effective tool to combat bacterial infection.
These findings are described in the article entitled Synthetic clay mineral as nanocarrier of sulfamethoxazole and trimethoprim, recently published in the journal Applied Clay Science. This work was conducted by D. Hernández, L. Lazo, L. Valdés, and A. Rivera from the University of Havana, L.C. de Ménorval from the Université Montpellier, and Z. Rozynek from the Adam Mickiewicz University.









