Since their discovery in 1981, stem cells have been touted as a kind of panacea for all conditions. The ability to individually program cells to perform whatever function needed would make most modern medicine obsolete as patients could instead be given cultures of healthy cells to fix their ailments. With the 2006 discovery by Japanese researcher Shinya Yamanaka that adult cells could be reverse engineered into multipotent stem cells, people envisioned a medical future where body parts, organs, and tissue could be custom grown from a culture of a patient’s own cells. That future may soon be a reality. Now, for the first time, clinicians from the University of Würzburg in Germany report having successfully grown functioning heart muscle from stem cells found in vascular walls.
In a study published in Circulation Research, the team reports how they stimulated the growth of novel cardiomyocytes in the hearts of mice by using a certain kind of stem cell. The select stem cells, called Flk1+CD34+ vascular adventitia-resident stem cells, were harvested from the walls of the aorta and are produced naturally in the body after a myocardial infarction (heart attack). Generally, regeneration of myocardial tissue after damage is extremely limited. Most existing stem cell treatments for heart attack are unable to stimulate new cardiomyocyte growth but only strengthen the existing tissue. The current study shows that it is indeed possible to stimulate endogenous stem cell repair of damaged heart tissue. “Our results provide a new approach, in that it may be possible to therapeutically manipulate the behavior of the stem cells in the intracardiac vascular walls so that they are stimulated into regenerating the destroyed cardiac muscle tissue,” lead author Süleyman Ergün told Würzburg University press.
How To Use Stem Cells To Do Things
Stem cells, in a nutshell, are biological cells that are able to develop into a wide variety of cell types; muscle cells, skin cells, hepatic cells, etc. Stem cells are kind of like a universal cell, capable of taking on whatever role they need to. In mammals, there are two broad classifications of stem cells. Embryonic stem cells are found in the embryonic tissue of a developing organism while in adult organisms, stem cells and progenitor cells are found around the body and assist with tissue repair. In general, embryonic stem cells are more potent and viable for differentiation than adult stem cells, but the ethical dilemmas surrounding the use of human embryos for medical research has caused scientists to look for ways to harvest stem cells from developed adults.
In 2006, Shinya Yamanaka demonstrated that adult fibroblast cells in mice could be transformed in pluripotent stem cells capable of turning into any cell lineage. This discovery kicked off an explosion into research on adult stem cells and their properties of cellular genesis. The current study builds on previous work to harvest and effectively use pluripotent stem cells found in adult tissue. During a heart attack, lack of blood flow can result in damaged and scarred cardiac tissue. The heart will naturally repair some of this tissue, but rarely enough to significantly improve the functioning of the heart post-infarction. Most of the time the damaged regions scar over and become inflexible and unresponsive to electrical stimulation.
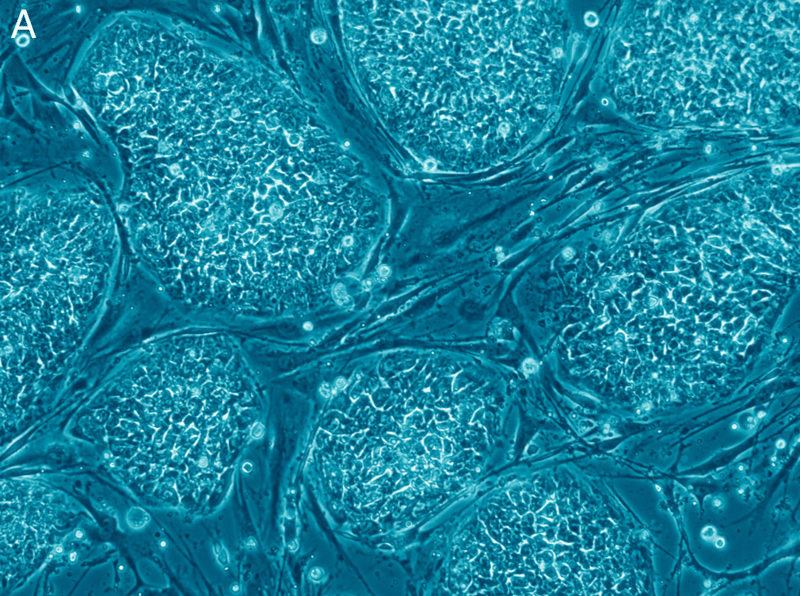
In order to stimulate cardiomyocyte growth, the team first needed to harvest the appropriate stem cells and progenitor cells from the vessel wall of the aorta. After removing the cells, the team cultured them in the lab to promote the growth of a cell colony. After about 10 days, beating cells spontaneously developed in the culture. The beating cells were examined and were determined to be functioning myocardiocytes, showing all the characteristic features of adult myocytes.
To test the potency of these cells in vivo, the team injected their cultured stem cells into the heart of a chick embryo. Within 4 days, they observed the tagged cells had differentiated into cardiomyocytes and had integrated themselves into the embryonic myocardium. Most importantly, the achieved this result without any genetic manipulation as the stem cells were naturally integrated into the chick embryo.
Although these types of vascular stem cells occur naturally in the body, they do not generally allow for significant repair of damaged heart muscle. The main problem is that endogenously, these stem cells are not potent enough to naturally repair the heart after it is damaged. Heart attacks also result in the death of endothelial and macrophage cells, both required for proper cardiogenesis. After an infarction, the body starts to release these kinds of stem cells for repair but they fail to differentiate and are incorporated into the heart as scar tissue. In their study, the researchers proved that it is possible to circumvent this natural limitation on the body’s healing capacities. Sca-1−CD34+Flk1+ stem cells found vascular walls have cardiogenetic properties that can work even in the face of post-infarction damage. Novel therapeutic treatments involving vascular stem cells have the potential to stimulate proper cell differentiation, resulting in the formation of functioning cardiomyocytes instead of scar tissue.
At this time, the researchers’ findings are limited to studies done on non-human animals in the lab but it is hoped that research will advance quickly enough to allow for therapeutic applications in humans. Most importantly, such techniques can be used to derive cultures from a patient’s own sample of cells. Stem cells recruited from other organisms can run the risk of being rejected by the patient’s body. On the other hand, stem cells derived from the patient themselves face virtually no risk of rejection and are always available for the body to use. Heart disease is a leading cause of death in the developed world for both men and women and existing cardiac surgeries always carry an inherent risk, so effective and safe procedures for treating heart damage are needed.









