
Almost six billion people around the world, mostly in the developing countries, are struggling every day to get a sufficient amount of non-hazardous water for household and agricultural uses. Waterborne diseases are the major cause of concern in the developing world, especially among the poor people who are living in unhealthy rural and slum areas, where the supply of purified water is not adequate.
The whole world is on the threshold of a water crisis, and the situation is going to be dreadful in the near future. Meanwhile, fresh water sources are also depleting very fast. In this scenario, the treatment of wastewater from domestic, agricultural, and industrial origins is of great importance to deal with the water crisis.
Reverse osmosis, UV purification, and slow sand filtration are among the popular techniques used nowadays. Slow sand filtration requires a large area to operate which is not feasible everywhere. Reverse osmosis is an immensely popular technique, mainly used in the household purposes, using four liters of wastewater to generate one liter of purified water. This is not acceptable, as it only enhances the water shortage issue due to its high waste ratio. Also, underprivileged people from the rural and slum areas of developing countries cannot afford these techniques because of their high operating cost.
Flocculation is a trivial yet very easy and cost-effective process to remove undesirable particles from wastewater. It can be used with small-scale (domestic) or large-scale (industrial) water treatment. It requires a very few apparatus and wastage of water is very minimal. 3 wt% kaolin suspension is taken as the model wastewater for all the experiments. This was prepared by dispersing 3 gm of kaolin in 100 ml tap water in a stoppered graduated cylinder of height 40 cm and inner diameter 2 cm. Kaolin forms a highly stable aqueous suspension, and the consumer industries (ceramics, cement, paint, paper filler, coating pigments, rubber additives, and extender for water-based paints and ink, etc) face a critical problem of kaolin removal from waste effluents. The high stability of kaolin suspension is due to its negatively-charged small particle size and anisotropic shape.
The flocculation efficacy of flocculants depends on many factors, like pH and ionic strength of the suspension medium; concentration, hydrodynamic size, conformation and functional groups of the flocculant molecule; and the size and density of the flocs generated during the process. Previous studies show that poly (vinyl alcohol) (PVA) (Mn 14,000) and Acacia nilotica gum extracts (NG) can act as an efficient and biodegradable flocculant for kaolin removal. Kaolin removal occurs very rapidly when PVA is used as a flocculant, but the clarity of the supernatant water is not very good.
In this investigation, NG is blended with high hydrolyzed grade PVA in different compositions to produce green and efficient flocculants for the separation of kaolin particles from its suspension. Flocculation was carried out using the classical jar test method. A 50:50 mixture of PVA and NG (PVA-NG55) was used at various concentrations (9 -35 ppm) at neutral pH (7.0) to determine the best flocculant dose. Both PVA and NG molecules adsorbed over the kaolin particles due the viscoelastic nature and hydrogen bonded interaction between both –OH and –COOH groups of the macromolecules and Si-OH groups of the clay particles. Therefore, the suspended particles come close to coalesce and settle under gravity.
It was observed that 25 ppm is the optimum flocculation dose considering both settling rate and turbidity of the supernatant (Table 1). The zeta potentials of both flocculants and kaolin suspension are negative, but due to the natural tendency of the macromolecules to adsorb over the solid particles, the degree of adsorption increases with increase in flocculants concentrations. As all the functional groups were likely to be in the ionized form, the hydrogen bonding also plays an important role. But after 25ppm, the degree of adsorption was decreased due to strong repulsive interaction among the flocculants and the colloidal particles and thus the settling time increased even after an increase in flocculant dose.
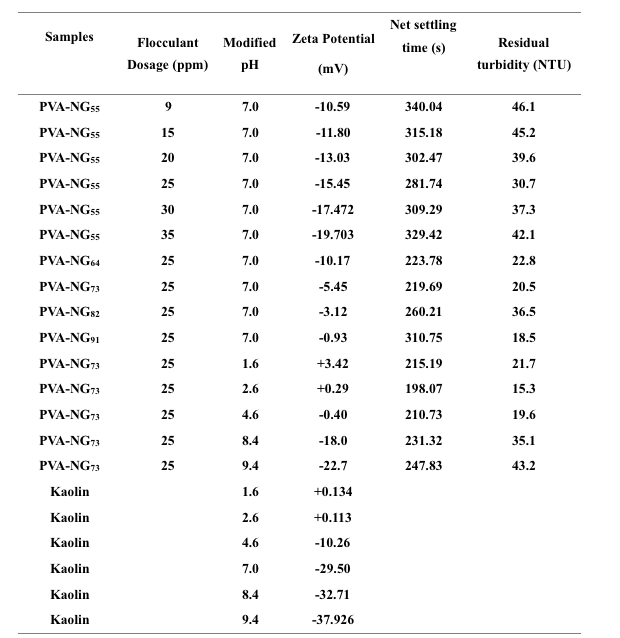
Table 1
Optimum blend composition is determined by carrying out the experiments using different compositions PVA-NG blends. PVA-NG73 shows the best result among the blends. Here, it is essential to look into the values of both reduced viscosity and zeta potential. The reduced viscosity of the blends increases with the increase in the concentration of PVA in the blends. The zeta potential gradually decreases with the reduction in NG concentration as zeta potential of neat NG is -19.4 mV at pH 7.0 and that of PVA is -0.095 mV. So, both high hydrodynamic volume and low zeta potential value enhance the settling process gradually from PVA-NG55 to PVA-NG73. Beyond that, these factors cannot explain the settling rate. This may be due to the fact that the ability to form hydrogen bonds is greater for –COOH groups of NG than -OH groups of PVA.
Effect of pH on the flocculation efficiency of PVA-NG73 was investigated as it can directly influence the zeta potential and hydrodynamic size. The efficiency was measured at the pH levels of 1.0, 2.0, 4.0, 7.0, 9.0 and 10.0 of the flocculant solution. On addition of 25 ml of PVA-NG73 of pH 1.0, 2.0, 4.0, 7.0, 9.0 and 10.0 into the suspension, the pH of the medium changed to 1.6, 2.6, 4.6, 7.0, 8.4 and 9.4 respectively. PVA-NG73 shows a better result as compared to neutral and basic regions. But, instead of pH 1.6, PVA-NG73 produces the best result at pH 2.6. Zeta potentials of PVA-NG73 at pH 1.6 and 2.6 are +3.42 and +0.29 mV respectively.
This high zeta potential value of PVA-NG73 at pH 1.6 reduces the hydrodynamic volume as high potential value twist the molecule to minimize due to intermolecular repulsion. Hence, PVA-NG73 gives the best result at pH 2.6.
The investigations are also carried out in presence of in presence of NaCl, CaCl2, and FeCl3. When either CaCl2 or FeCl3 was added, the hydrated metal ions adsorbed over the kaolin surface and decrease the double layer charge density close to zero. Thus, the suspension was already destabilized before addition of any flocculants. When PVA-NG73 was added, it formed bridges between the already de-stabilized kaolin particles and increases the settling rate. But, this happens in up to 0.03 M concentration. After 0.03 M concentration, the settling rate slows down due to steric stabilization. The inactivity of NaCl is may be due to the low charge density of Na+.
This study revealed that PVA-NG can be used as a cheap, effective and green flocculant. 70:30 PVA-NG blend shows the best result at pH 2.6. This efficiency can also be enhanced by adding either CaCl2 or FeCl3.
These findings are described in the article entitled Flocculation of aqueous kaolin suspension using a biodegradable flocculant system of ply (vinyl alcohol)- Acacia nilotica gum blends, recently published in the journal Applied Clay Science. This work was conducted by Tanbir Nasim, Abhijit Pal, and Abhijit Bandyopadhyay from the University of Calcutta.









