
Mitochondria are tiny bodies inside the cells of animals, plants, and fungi that were derived from an endosymbiotic process involving bacterial and archaeal cells. Despite their tiny size, mitochondria are essential for energy production, stress response, and other important biochemical pathways. These processes make them look like busy factories, but, just like any other factories, with bigger productions come bigger waste.
To do their regular activities, mitochondria mostly do not produce their own proteins. Most of them are synthesized outside the organelle and have to be imported inside with the help of a sorting signal that usually resides in the N-terminal targeting peptide. After precursor proteins are imported, a targeting peptide is cleaved by the processing peptidase and released into the mitochondrial matrix as by-products. As with any other waste, a free-targeting peptide could be dangerous if not handled properly.
In humans, the accumulation of free peptides has been associated with many diseases. An infamous example is the accumulation of amyloid beta-peptide, which causes Alzheimer’s disease. In plants, peptide accumulation was associated with disrupted organellar stability, as they can strongly interact and disrupt the lipid membrane. Free peptides can also disturb the protein import process, as they may bind to processing peptidases and trigger a premature stress response.
Mitochondria have evolved ways to manage this waste problem. One of the mechanisms that has been elucidated in great detail is the processing pathway, which was first fully characterized in plants.
Contrary to the proteasomal degradation in the cytosol, which is a straightforward degradation of ubiquitinated proteins or peptides by the proteasome, processing pathways require a stepwise degradation by multiple different peptidases. Long free targeting peptides are fragmented into oligopeptides by preprotein peptidase (PreP). Oligopeptides are then further degraded by organellar oligopeptidase (OOP) into short peptides. Finally, single amino acids are released from short peptides by aminopeptidases.
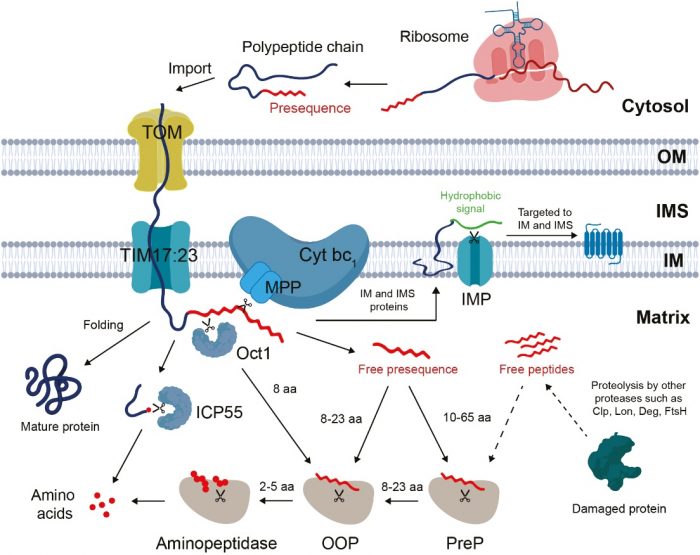
Figure. The processing pathway of protein import in plant mitochondria. Figure courtesy Abi Sofyan Ghifari.
Interestingly, this peptide-degrading mechanism is shared between mitochondria and another endosymbiotic organelle in plants, the chloroplast. Most of the peptidases involved in the processing pathway, such as PreP, OOP, and aminopeptidases, are dually-targeted, meaning that these peptidases are targeted and localized to both mitochondria and chloroplasts. Inactivation of these peptidases altered organellar stability and activity, which overall affects plant growth, development, and stress response.
As plants have hundreds of different proteases and peptidases, it is possible that other proteins may be involved in the process, which is essential for plant growth and response to environmental conditions.









