
Throughout the history of homo sapiens, there has been an intense focus and investment on alleviating the signs and symptoms of aging whether it be the treatment of wrinkles, hair coloring, hair restoration, or devising novel ways of longevity.
Count no mortal fortunate till he has departed this life free from pain – Sophocles
The current industry of anti-aging drugs and procedures is worth billions of dollars and is expected to rise to $210 billion by the year 2021. For longevity itself, many religious and social ways of life have been adopted by various individuals, yet to date, no human trials have been completed showing an increase in longevity with interventions.
One must remember that with longevity comes a price – an increase in age-related conditions which include cancers, dementia, heart failure, frailty, and psychologic morbidities. Numerous factors play a role in aging including lifestyle factors (smoking, diet, exercise, and alcohol intake are the top four), environmental factors, socio-political factors, and genetic factors. Though it is hard to completely separate out normal versus abnormal aging with respect to both biology and phenotypic changes, observational studies over decades have clearly demonstrated that certain intrinsic and extrinsic factors can affect the aging of a cell.
Topping this list of agents is chemotherapy and radiation, which induce a state of cellular changes which eventually lead to accelerated aging phenotypes, compromising both the quantity and quality of life of individuals. In this review, I will explain the basics of accelerated aging in patients who have survived cancers and then possible solutions to avoid pathologic aging at the tissue level or clinically.
Currently, cancer management has seen a major shift in diagnosis and treatment compared to half a century ago; though cancer research started in an organized manner in the 1930s (the National Cancer Institute was the first institute of the NIH and was established in 1937), it got a significant push when President Nixon declared a “war on cancer” by signing the National Cancer Act in 1971. Since then, just within the past 4 decades, we have seen a tremendous growth in clinical trials for cancer treatments which have led to a better survival.
Now, the majority of childhood cancer survivors and more than 2/3rd of adult cancer survivors are living beyond 5 years of receiving a cancer diagnosis. Some diseases have had such remarkable treatment successes that they have been declared a chronic disease e.g. chronic myeloid leukemia has excellent treatments currently with oral chemotherapy pills (“Imatinib” and sister drugs) leading to a 10-year survival of 85% or more.
Traditionally, the oncology community has focused on acute short-term toxicities (alopecia, nausea/vomiting etc.) but as the cancer survivors are crossing the 5-year mark, delayed toxicities of chemotherapy and/or radiation are becoming apparent. Heart failure, dementia, frailty, and new cancers, which are the hallmarks of geriatric populations, are appearing much earlier in the cancer survivors, even in their middle ages (especially in pediatric cancer survivors).
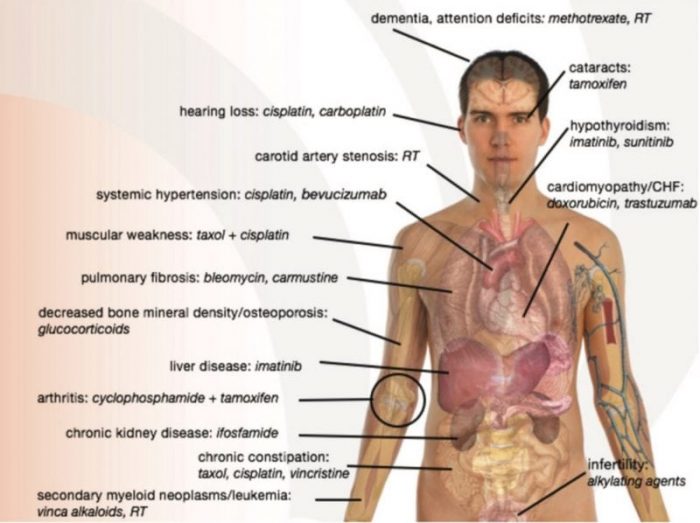
A depiction of age-related effects of respective cancer therapies. Reprinted with permission from the BMJ Journal.
Though observational studies from large databases (especially from Children’s Oncology Group [COG] and the Center for International Blood and Marrow Transplant Research [CIBMTR]) have clearly shown an increase in these age-associated manifestations, recently many groups have demonstrated the biology behind this phenomenon. Wood et al. demonstrated that the senescence of human immune system (measured by the marker p16INK4a), increases following a stem cell transplantation. Quintela-Fandino et al. recently demonstrated that the percentage of critically short telomeres in cells from breast cancer patients is determined by the chemotherapy toxicity.
Li et al. have demonstrated that chemotherapies with different mechanisms of action can lead to significant shortening of telomeres, down-regulation of telomerase activity, and diminished expression of telomerase reverse transcriptase (hTERT). Cupit et al. recently summarized the mechanisms behind the clinical aging phenotypes and proposed mechanistic approaches in how to potentially prevent these complications. Impaired DNA damage responses, stem cell exhaustion, telomere shortening, accelerated accumulation of senescence cells are some of the mechanisms which are being evaluated with mitigation strategies in cancer survivors currently.
One potential solution comes from studies arising from the Kirkland Lab (Mayo Clinic) in which drugs which can alleviate the burden of senescent cells in animals can potentially be utilized to study their effect on human tissues from cancer patients. Another approach can utilize the role of mTOR inhibitors which have shown to delay the senescence phenotypes in animals. SASP modifiers have also been used in animals with encouraging data to alleviate signs of aging in animal models. More important is to develop more effective anti-cancer treatments with less late toxicities.
In the cancer management paradigm, we are seeing a major paradigm shift from chemotherapies to cellular therapies (especially chimeric antigen receptor T-cells [CAR-T]) and immunotherapies (especially cancer vaccines and checkpoint inhibitors), however, the long-term toxicity data from these novel therapies is unavailable, and we need to be extremely cautious with close surveillance of the patients receiving these types of treatments. Lastly, Cupit et al. emphasize that there is an essential need for follow up of these cancer survivors lifelong ideally in a long-term follow up clinic lifelong.
I find Shakespeare’s quote, “all’s well that ends well,” most applicable to the millions of cancer survivors worldwide since the whole purpose of cancer treatment is to give life rather than prolonging life with sufferings. Thus, once a cancer patient receives chemotherapy or radiation, the utmost efforts should be undertaken for early recognition of the known age-associated complications of the cancer treatments with close follow up in clinics and screening strategies.
The principle of utilizing drugs to alleviate senescence burden in cancer survivors is not longevity; in fact, the principle of saving lives of cancer patients has never been to achieve a centenarian status. Combating or preventing multiple aging-associated diseases in long-term cancer survivors is the goal of evaluating anti-aging measures so that the quality of life is maintained till the end. I believe that the studies mentioned above have established a strong foundation for testing anti-aging strategies in cancer survivors, which, if left in limbo, are bound to suffer from many age-associated illnesses.
These findings are described in the article entitled Biology of premature ageing in survivors of cancer, recently published in the journal ESMO Open. This work was conducted by Margaret C. Cupit-Link, James L. Kirkland, Tamar Tchkonia, Nathan K. LeBrasseur, Kathryn J. Ruddy, and Shahrukh K. Hashmi from the Mayo Clinic, Kirsten K. Ness and Gregory T. Armstrong from St. Jude Children’s Research Hospital, and Saro H. Armenian from the City of Hope National Medical Center.









