
For years, the appendix has been thought of as mostly useless, an insignificant piece of tissue tacked on to the end of the large intestine whose only claim to notoriety was becoming inflamed to the point of bursting.
But now, research published by my lab and our collaborators in the U.S. and Sweden points to a surprising new role for the appendix — as a possible starting point for Parkinson’s disease, a neurodegenerative disorder that progressively robs a person of their ability to move and brings with it a host of other symptoms, such as depression, gastrointestinal issues, fatigue, and loss of sense of smell.
We found that the appendix is a reservoir for abnormally shaped alpha-synuclein proteins, which are a hallmark of Parkinson’s and are linked to the gradual destruction of brain cells that underlie the disease’s symptoms. These proteins, which can form aggregates called Lewy bodies, travel from neuron to neuron, clogging up the machinery required to keep cells healthy and functioning. As cells die off, the brain becomes starved of dopamine, a neurotransmitter that relays important messages about voluntary movement and a litany of other day-to-day tasks.
Our work shows that removing the appendix early in life — and by extension, the alpha-synuclein contained within — reduces risk of developing Parkinson’s by about 19 percent in the general population and up to 25 percent in rural populations. This variation may be due to the appendix’s responsiveness to potential environmental triggers of Parkinson’s (for example, people who live in rural areas often have a higher incidence of Parkinson’s possibly due to increased pesticide exposure). For those who go on to develop Parkinson’s, removal of the appendix can delay the onset of symptoms and push diagnosis back by an average of 3.6 years. The protective effect did not extend to people whose disease is directly caused by a genetic mutation — a group that comprises fewer than 10 percent of all cases.
In another stunning turn, we found abnormal alpha-synuclein in people of all ages and with and without Parkinson’s, raising new questions about the mechanisms that give rise to and propel the disease. Previously, abnormal alpha-synuclein was thought to occur in people with Parkinson’s or Parkinson’s-related dementia.
It now appears that, while these pathogenic forms of alpha-synuclein are toxic in the brain, they are normal in the appendix. Only about 1 percent of the population develops Parkinson’s, meaning that the presence of alpha-synuclein alone is not enough — there must be other factors or confluence of factors at play that trigger disease onset, such as environmental exposures or genetic mutations.
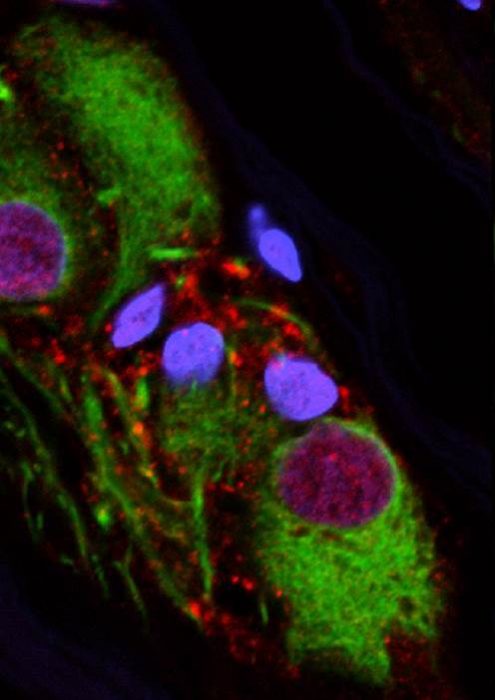
Clumped forms of alpha-synuclein proteins are a key feature of Parkinson disease, but new research shows that nerve cells of the human appendix also hosts an abundance of clumped alpha-synuclein. Appendix nerve cells are colored in green, clumped alpha-synuclein is colored in red, and nuclei of appendix nerve cells are colored in blue. This suggests that clumped alpha-synuclein is normally present in the appendix but if it were to leave and enter the brain it could trigger Parkinson’s disease. Image courtesy of the Labrie Laboratory | Van Andel Research Institute
There are some important caveats to our findings, however. In order for people to experience the protective effect of an appendectomy, the appendix must be removed at a relatively young age, before the disease process has begun. This window of time could be decades before the first appearance of symptoms and may vary widely from person to person.
It’s also important to remember that removing the appendix mitigates risk but does not eliminate it. Parkinson’s is a challenging disease to study and treat because its genesis likely lies in a web of intertwined factors, such as genetic, epigenetic and environmental influences. Removing or reducing one of these elements — such as the alpha-synuclein in the appendix — can help but may not totally protect someone from the disease.
There’s also the matter of the appendix itself. We still don’t exactly understand its role in the body, but growing evidence suggests that it may be more valuable than previously thought. For example, we now know the appendix is an important player in the immune system and acts as a microbial warehouse for the intestine, capable of repopulating the gut’s microbiome if it is damaged or wiped out. As such, removing it as a preventative measure for Parkinson’s just isn’t a good approach and could have effects down the line that we can’t foresee.
Instead, reducing levels of these Parkinson’s-related proteins in the gut may be our best bet. We envision a future where people may be screened for Parkinson’s risk and then treated with a medication that dampens down levels of these harmful proteins, delaying or even stopping their journey to the brain. There are experimental medications are currently in clinical trials that are designed to do just that, and we look forward to the results of these studies.
New treatment methods are desperately needed. Currently, we have no effective way to slow or stop progression, only to treat symptoms. But even still, the gold standard therapy, levodopa, can have challenging side effects when used long-term, such as uncontrolled movements called dyskinesias and even hallucinations. It is imperative that we look beyond the brain for ways to improve the quality of life for people battling this devasting disease. With the evidence presented by our study and others, we believe the gut is the best place to start.
These findings are described in the article entitled The vermiform appendix impacts the risk of developing Parkinson’s disease, recently published in the journal Science Translational Medicine.









