
Recently, news such as the story of a polar bear desperately reaching for an ice floe and a global-scale coral bleaching have become increasingly common. With climate change, extreme weather events are clearly increasing in frequency, such as intense heatwaves, powerful hurricanes, or severe droughts.
These effects do not only appear on the surface of the earth but also beneath the ocean, with harmful effects on various marine ecosystems. For example, ocean acidification (OA), which known as the evil twin of global warming, is one of the emerging threats to marine environments (Feely et al., 2004).
OA, which is caused when water absorbs 30% of atmospheric carbon dioxide (the earth absorbs about only 20 %), should be considered seriously. Experts have predicted a drop in seawater pH by 0.3-0.4 units by the end of the century and concluded that the average oceanic pH will decrease by 0.8 units by 2300.
Why should people worry about this number, since it seems so small? A point should be made — that pH is a logarithmic scale, so 1 pH unit different means 10 times the amount of hydrogen ion concentration, so only 1 unit of pH change can cause catastrophic consequences for marine organisms (Fig. 2).
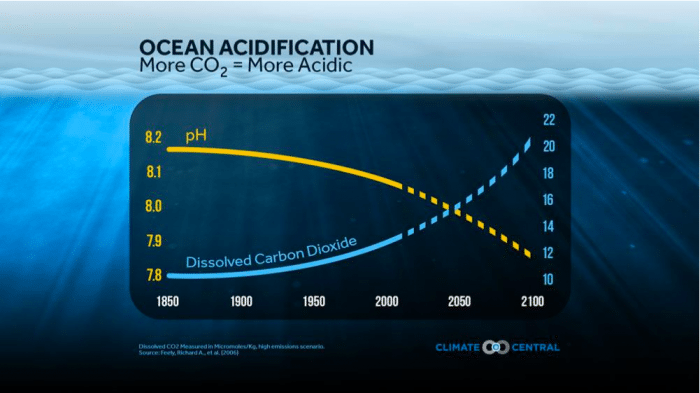
Fig.2 Dissolved CO2 measured in micromoles/Kg. high emissions scenario. Source: Feely et al., (2006)
Note that OA not only directly impacts shell-forming organism fitness through reduced calcification rates but also through changes in reproduction, physiology, growth rate, and behavior. So, it’s of great significance to understand the adaptive mechanisms of marine organisms to cope with environmental stress such as OA. On the other hand, decreased pH could alter the physiochemical properties of nanomaterials, leading to a slightly greater aggregation similarity in size and shape to phytoplankton. This phenomenon is a hazard to filter feeders such as mussels. Titanium dioxide nanoparticles (nano-TiO2), one of the most extensively-used metal oxide nanoparticles in industrial products such as sunscreen, was the preferred choice to further clarify the interaction of toxicity impacts with low pH condition relevant for ocean acidification.
These findings suggested nano-TiO2 (2.5, 10 mg L−1) and low pH (pH 7.3) induced oxidative stress in the thick shell mussel Mytilus coruscus and of that with interactive and carry-over effects. That is to say, the combined effects of nano-TiO2 with low pH on biochemical parameters of M. coruscus not only can be observed during an exposure period but can also continue to be detected during the recovery period. What should be highlighted are the tissue-specific responses: the mussel digestive gland was able to avoid oxidative stress. This aspect of the experimental results showed an increase in some antioxidant enzyme activities. On the contrary, the gill seems to be more sensitive to oxidative stress and might undergo damage originating from high-concentration nano-TiO2 exposure due to low dissolution rate under low pH condition. Furthermore, the hemolymph is also a target organ. Our previous study represented the first evidence of suppressive immune response when mussel hemocyte was exposed to combined nano-TiO2 and low pH (Huang et al., 2016).
In the second part of this research, followed by a short-term recovery period under normal pH (pH 8.1) without nano-TiO2, both tissues of M. coruscus were not sufficient to fully restore the damaged antioxidant system; that is, mussels could not adapt effectively. From the viewpoint of individual growth, other previous studies found that physiological functions of M. coruscus were more severely impaired by the combination of nano-TiO2 and low pH, strongly correlating to a negative respiration rate, ammonia excretion rate, O:N ratio, and scope for grow (SFG) values (Hu et al., 2017). Taking into account all the findings, we could confirm that nano-TiO2 exposure poses adverse effects in M. coruscus, especially impacting mussel health more seriously when seawater acidification was present (Fig. 3).
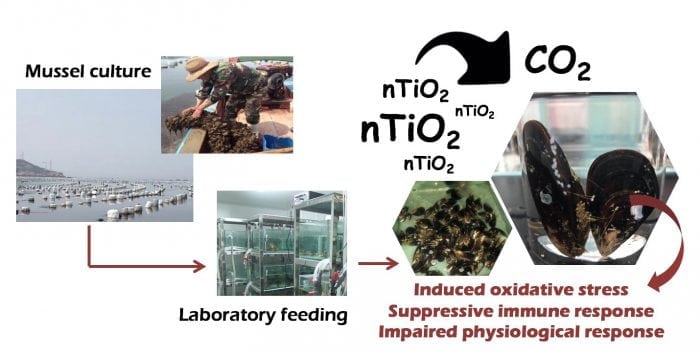
Credit: Xizhi Huang
Although this work may not reflect realistic environmental exposure conditions, results from this study can provide evidence of sublethal negative effects of nano-TiO2 and low pH on coastal marine ecosystem engineers. The synergistic effects of nano-TiO2 and low pH on mussels suggest that nanoparticles may affect marine invertebrate fitness more severely when additional environmental stressors are present.
These findings are described in the article entitled Oxidative stress induced by titanium dioxide nanoparticles increases under seawater acidification in the thick shell mussel Mytilus coruscus, recently published in the journal Marine Environmental Research. This work was conducted by Xizhi Huang, Zekang Liu, Zhe Xie, Fangli Wu, Hui Kong, Liping Liu, Yanming Sui, Weiqun Lu, Menghong Hu, and Youji Wang from Shanghai Ocean University, Sam Dupont from the University of Gothenburg, Wei Huang from the Second Institute of Oceanography State Oceanic Administration, and Daohui Lin from Zhejiang University.
References:
- Feely, R.A., Sabine, C.L., Lee, K., Berelson, W., Kleypas, J., Fabry, V.J., Millero F.J. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 305, 362-366.
- Feely, R.A., Sabine, C.L., Fabry, V.J., 2006. Carbon dioxide and our ocean legacy. Mrine Life. 1.
- Huang, X., Lin, D., Ning, K., Sui, Y., Hu, M., Lu, W., Wang, Y., 2016. Hemocyte responses of the thick shell mussel Mytilus coruscus exposed to nano-TiO2 and seawater acidification. Aquat. Toxicol. 180, 1–10.
- Hu, M., Lin, D., Shang, Y., Hu, Y., Lu, W., Huang, X., Ning, K., Chen, Y., Wang, Y., 2017.CO2 -induced pH reduction increases physiological toxicity of nano-TiO2 in the mussel Mytilus coruscus. Sci. Rep. 7.









