
Soft polymers are an integral part of our daily lives, ranging from natural biopolymers such as proteins, DNA, and polysaccharides found in living systems, to commercialized synthetic polymers such as plastics, fibers, and elastomers. In general, these conventional polymers are either significant insulators or dielectrics.
Since the pioneering discovery of metallic conductivity in doped crystalline polyacetylene by Shirakawa in 1977, for which he was awarded the Noble prize in Chemistry in 2000 together with Heeger and MacDiarmid, we have witnessed an unprecedented rise in the popularity of conducting polymers. Conventional organic polymers possess beneficial properties such as flexibility and structural diversity, while conducting polymers introduce optical and electrical/electronic properties, making them ideal for diverse applications in energy production, electrochromic devices, sensors and actuators, bioengineering, and biomedical applications.
Poly(3,4-ethylenedioxythiophene) (PEDOT) is one of the most promising and successful conducting polymers with excellent stability, reversible doping state, high conductivity, and electrochemical properties. However, PEDOT was initially considered to be an insoluble polymer, limiting its ability to be processed as a solution for large-scale device fabrication. The solubility problem was subsequently solved by using a water-soluble anionic polyelectrolyte, poly(styrene sulfonic acid) (PSS) as the charge-balancing dopant during polymerization to yield the water-soluble PEDOT:PSS composite, which has emerged from the laboratory to form the basis of a range of commercial products for antistatic, electronic and bioelectronic applications.
While circumventing the solubility issue, the relatively high molecular ratio of PSS and its sulphonic acid (SO3–) moiety endows PEDOT:PSS with decreased conductivity and a strongly negatively-charge. A research team at the Linköping University, Sweden recently developed a new type of solution-processable and positively-charged PEDOT colloids prepared through a template-assisted polymerization, avoid the utilization of insulating PSS and result in a polymer with a positive surface charge by virtue of the oxidized form of the PEDOT polymer backbone.
The recent emergence and rapid development of printed electronics and soft electronics present new challenges and places huge demands on new conductive and semi-conductive materials. Finding new conductive and semi-conductive materials is essential to boost the growth of the global printed electronics industry and consequently, the market it serves. The development of printed electronics will also benefit the area of flexible sensors, medical wearable patches, organic light-emitting diode (OLED), and organic photovoltaic (OPV). As a key component for printed electronics, solution-processed PEDOT inks could combine with several industrial-scale printing techniques (e.g. screen-printing, inkjet-printing, and spray-coating) for high throughput large-scale fabrication of different electrodes, electronic, and biosensing devices.
In the field of electrocatalysis, the surface charge of conducting polymer electrodes greatly influences the efficiency and kinetics of the electrocatalytic process at the reactant-electrode interface, due to the mass transfer kinetics and electrostatic accumulation of the reactants. Nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADHP) are the key redox reactants for bioelectrocatalysis and biosensors since they are coenzymes found in the human body that participate in reactions with more than 300 enzyme dehydrogenases and are an essential adjunct to electron transport chain in photosynthesis.
Mak’s team at Linköping has shown that these solution-processable PEDOT inks can be used as building blocks for the fabrication of a hierarchically-structured and positively charged electrode with substantially enhanced electrode kinetics for NADH oxidation, which is facilitated by the increased active surface area of the electrode and electrostatic accumulation of the negatively-charged NADH on the PEDOT electrode surface.
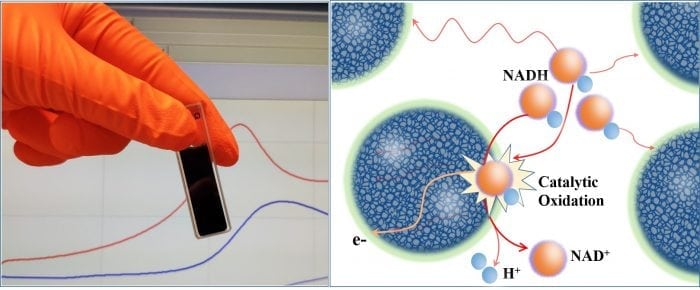
Solution-processable PEDOT colloidal inks and its catalytic oxidation of NADH (Credit: Martin Wing Cheung Mak)
In addition to the NADH electrooxidation, by coupling the PEDOT-based NADH electrochemical transducer with dehydrogenase enzymes, it is possible to develop biosensors for a wide range of analytes (e.g. glucose, alcohol, formate, aldehyde, lactate, β-hydroxybutyrate etc) through the detection of NADH generated from the specific enzyme reaction. Stable and manufacturable catalytic electrodes for NADH oxidation are an essential prerequisite to realizing multi-parameter metabolite sensors for applications in clinical diagnostics and other life science applications, as well as underpinning the development of dehydrogenase-based biofuel cells, bioreactors and biocatalytic processes.
These findings are described in an article entitled Positively-charged hierarchical PEDOT interface with enhanced electrode kinetics for NADH-based biosensors, recently published in the journal Biosensors and Bioelectronics. The work was conducted by Lingyin Meng, Anthony P.F. Turner, and Wing Cheung Mak from the Department of Physics, Chemistry and Biology, Linköping University, Sweden.









