
Antibiotics once seemed like a miracle cure for infectious diseases. The time between the 1930s and 1970s was the pinnacle of the antibiotic discovery era. But since then, antibiotics development has dwindled. What happened?
In 1928, the biologist Alexander Fleming returned to the lab from holiday. He began to sort through petri dishes containing Staphylococcus bacteria and came upon one plate that was contaminated with a blob of mold. Upon closer examination, the zone immediately surrounding the mold was clear of bacteria, as if the mold had stopped the bacteria from growing. Fleming had serendipitously discovered penicillin.
In the decades to follow, antibiotic development became a focal point in the pharmaceutical industry. It was easy to chemically modify existing drugs like penicillin to make more effective drugs. When bacteria develop resistance to one drug, we could quickly found an alternative. But at some point, it becomes harder to make new drugs, harder to modify the already existing drugs.
In the 1990s, antibiotic development plunged for those reasons as antibiotic resistance has been on the rise due to overuse. Now we are facing an impending “post-antibiotic era,” a time where once treatable infections are untreatable, a time where the antibiotics we currently have are no longer effective due to antibiotic resistance, and a time where we have run out of new and effective antibiotics.
The need for new antibiotics remains great, but drug companies have no interest in antibiotic development. Why not? The answer is economics.
For many reasons, antibiotic development is not the way to the big bucks – antibiotics bring in less revenue once they are on the market compared to other drugs.
They cost the same to develop as drugs for chronic illnesses. When we have a bacterial infection, we go to the doctor and end up at the pharmacy to pick up a course of antibiotics. We take the antibiotics for a week or two and then we are done with them. On the other hand, drugs for chronic illnesses require medication continually for years, providing a constant stream of revenue to the drug developers.
But antibiotics have a bigger problem than their short treatment time: bacteria become resistant to antibiotics, sometimes in the matter of months or years. As an example, most Staphylococcus aureus strains isolated in British hospitals were resistant to penicillin a mere six years after the introduction of the drug. Another example: methicillin. Methicillin was introduced in 1959 to treat Staphylococcus aureus infections that were resistant to penicillin. Soon, in 1961, reports of methicillin-resistant S. aureus began appearing. When it takes so much time and cost for development, testing, and approval, antibiotic development is risky from a profit-oriented standpoint.
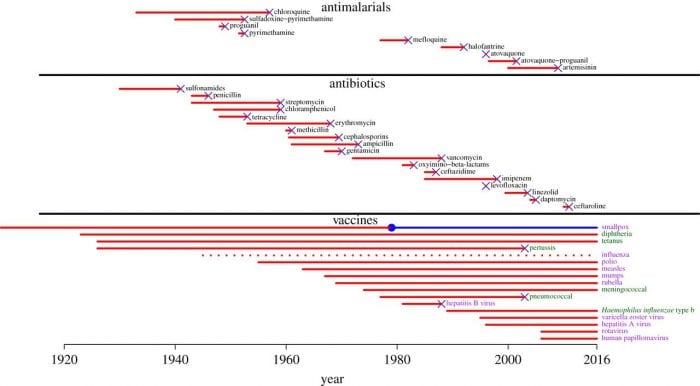
Timeline showing antibiotic introduction (leftmost point of red line) and the development of resistance to that drug (‘x’ at the end of the red line). Image credit: Kennedy and Read, 2017.
Lastly, the principle behind effective antibiotics is quite contrary to the principles behind the business. How do we prevent antibiotic resistance and ensure that they remain effective? By not using them. We only want to save them in the most desperate circumstances. “For antibiotics, business models based on sales volumes tend to promote overuse and thereby resistance,” writes a team lead by Kevin Outterson who studies health law at Boston University and is executive director of CARB-X, BU’s antibiotic accelerator. In the profit-driven world of pharmaceuticals, antibiotic discovery or development may not justify large investments of time and funds.
With these obstacles, how can we encourage antibiotics development from pharmaceutical companies?
Outterson and colleagues suggest that financial gains from antibiotics should reflect more than just sales volume. “Instead, companies producing a truly novel antibiotic should receive rewards after receiving marketing approval, linked not to sales but to R&D costs invested or potential therapeutic value,” writes Outterson and colleagues. When the economic incentive is “de-linked” from sales outcomes, innovation and not sales volume is rewarded.
Of course, saving the world from an antibiotic apocalypse requires concerted efforts from those in drug development, medicine, and agriculture. But without new antibiotics added to our arsenal, we may reach the post-antibiotic era much sooner than we like.









