
Since its identification as the cause of the Acquired ImmunoDeficiency Syndrome (AIDS) in 1983, the Human Immunodeficiency Virus (HIV) has received great attention, and not just from the scientific community.
Everybody knows “Philadelphia,” that wonderful movie with Tom Hanks and Denzel Washington, or “Dallas Buyers Club,” featuring Matthew McConaughey and Jared Leto. These are just a few examples of films dedicated to the AIDS epidemic, a media phenomenon also powered by the deaths of the famous singer Freddy Mercury and surrealistic painter Keith Haring.
Nowadays, there are almost 37 million patients with HIV worldwide and more than 2 million new infections per year globally. Each year, more than one million people are dying from an AIDS-related disease. Thes statistics show just how serious the AIDS problem still is after 35 years.
The current AIDS treatment regimen relies on a cocktail of drugs, referred to as combination antiretroviral therapy (cART), which improves the quality of life of patients who are HIV positive. However, the benefits of cART are often compromised by poor patient compliance, drug resistance, and by the inability of drugs to reach sanctuary sites and latently-infected cells. For these reasons, an urgent need to find new drugs is perceived.
The next generation anti-HIV therapy should focus on targets not currently reached by commercially-available drugs. In line with this, attention has focussed on structures whose inhibition could result in the shutdown of more than one step within the viral replicative cycle, thereby reducing the likelihood of resistance emergence while simplifying the treatment regimen. Among the viral structures that meet these criteria, the Nucleocapsid Protein 7 (NCp7) is worth a mention because of its importance in the HIV replication cycle. NCp7 (Figure 1) is a small, highly basic, zinc-containing protein that is essential for multiple stages of the viral replicative cycle and whose structure is preserved in all viral strains, including clinical isolates from therapy-experienced patients.
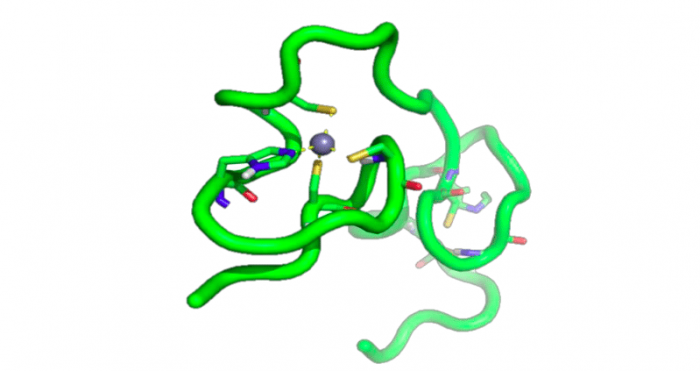
Figure 1: NCp7 structure
A number of inhibitors are known, but none of them have reached the market, even though some were evaluated in clinical trials. In our review articles [1,2], we detailed all the NCp7-inhibitors developed until now, detailing their mechanism of action and highlighting the structure-activity relationship of each class. The anti-NCp7 agents were clustered into three groups: covalent inhibitors, noncovalent inhibitors, and nucleic acid (NA) binders. Covalent NCp7 inhibitors, also referred to as irreversible inhibitors and/or zinc ejectors, were the first reported during the late 1990s, and their development was meant to discover compounds able to recognize NCp7 among cellular proteins containing zinc finger domains. The noncovalent class of NCp7 inhibitors is endowed with a weaker antiviral potency when compared with covalent binders and none of them ever reached clinical trials. Also, NAs binders are known, even if the importance of NAs in anti-HIV drug discovery goes beyond the exclusive inhibition of NCp7 functions.
The main aim of these articles is to equip the medicinal chemistry community with a solid drug design platform that can facilitate the development of a new generation of clinical, approvable NCp7 inhibitors. The newest NCp7 covalent inhibitors developed are the DISelenoBisbenzAmides (DISeBAs, Figure 2).
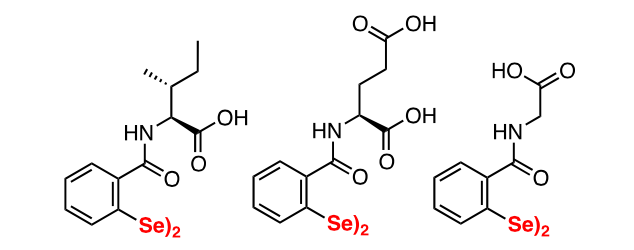
Figure 2: Structures of DiSeBAs
DISeBAs were developed in 2015, following a “me-too” approach, and are the sole NCp7 inhibitors characterized by the presence of selenium in their structure [3]. DISeBAs showed a potent antiviral activity measured in cells in the low micromolar range, coupled with no sign of toxicity. The antiviral activity also encompassed clinical isolate HIV strains from cART-experienced patients. This work is significant since it is the first example in which the pharmacological properties of selenium are successfully employed in anti-HIV agents.
These findings are described in the article entitled, NCp7: targeting a multitasking protein for next-generation anti-HIV drug development part 1: covalent inhibitors, recently published in the journal Drug Discovery Today. This work was conducted by Luca Sancineto from the Centre of Molecular and Macromolecular Studies, Nunzio Iraci from the University of Salerno, and OrianaTabarrini and Claudio Santi from the University of Perugia.
References:
- L. Sancineto, N. Iraci, O. Tabarrini, and C. Santi, Drug Discov. Today 23, 260 (2018).
- N. Iraci, O. Tabarrini, C. Santi, and L. Sancineto, Drug Discov. Today 23, 687 (2018).
- L. Sancineto, A. Mariotti, L. Bagnoli, F. Marini, J. Desantis, N. Iraci, C. Santi, C. Pannecouque, and O. Tabarrini, J. Med. Chem. 58, 9601 (2015).









