CD4 T lymphocytes are T-helper cells that harmonize the immune system to avert pathogenic infection. They do so by recruitment and activation of other immune cells, such as B-cells, macrophages, neutrophils and basophils. Weakening and damaging of the immune system by the impediment of this helper T-cell function is the focal cause of disease progression through HIV (Human Immunodeficiency Virus) infection.
AIDS (Acquired Immunodeficiency Syndrome) is the advanced stage of the disease associated with vulnerability towards opportunistic infections and/or development of cancer. There is no cure or vaccine for HIV or AIDS. The sole treatment, through ART (antiretroviral therapy) or HAART (highly active antiretroviral therapy), reduces the disease progression by decreasing the viral load (amount of virus in an infected person’s blood) and increases the life expectancy of the infected individual.
HAART, a combination therapy introduced in mid-1996, is mainly comprised of nucleoside/nucleotide reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors, integrase inhibitors, fusion inhibitors and entry inhibitors. Early detection and the right combination of these drugs can prevent the progression of HIV infection intoAIDS. On the downside, costly HIV medication is not accessible in resource-limited countries, viral resistance develops towards HAART and drug interaction predisposes to cardiovascular or neurological diseases. Therefore, it is necessary to develop novel small-molecule inhibitors, which are affordable and will act on resistant HIV strains without adverse side effects.
Nucleocapsid protein 7 (NCp7), a highly basic 55-residue protein, has been emerging as a promising target for next-generation antiretroviral drugs due to its essential function in viral replication and less prone to acquire mutations for viral resistance.
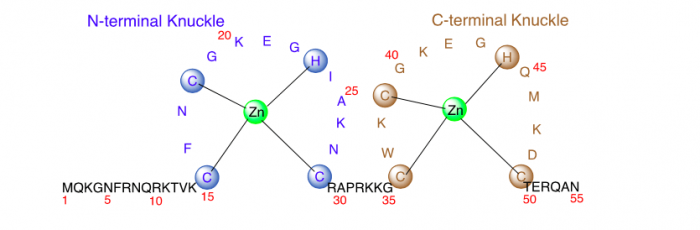
Figure 1. The primary sequence of Nucleocapsid protein 7 (NCp7) of the human immunodeficiency virus (HIV). Image published with permission from Wiley from https://doi.org/10.1002/cmdc.201700141.
Mercaptobenzamides (MB) based molecules have been shown to inactivate NCp7 by unfolding of the protein and leading to the formation of immature non-infecting HIV virions. The unfolding of protein occurs due to an acetyl transfer from the thioester group of MB to the nucleophilic cysteine residue of the protein, which subsequently leads to zinc ejection from the core and losing its active three-dimensional structural conformation.
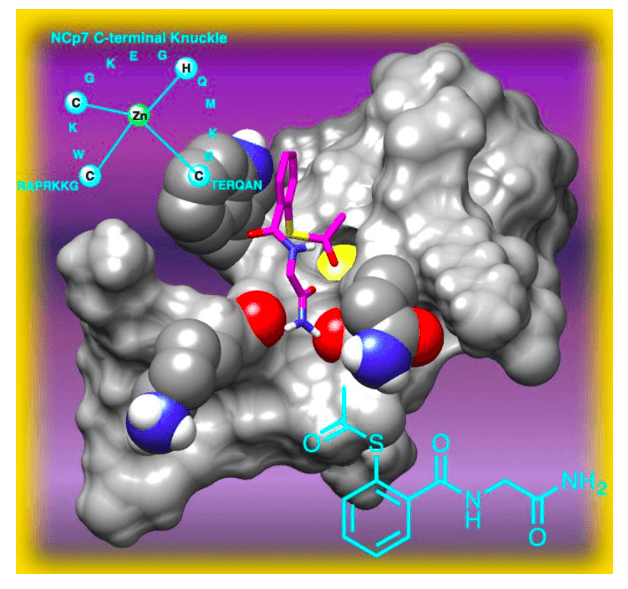
Figure 2. Probable mode of interaction between S‐acetylmercaptobenzamide and the C‐terminal domain of Nucleocapsid Protein 7 (NCp7) of the human immunodeficiency virus (HIV). Image published with permission from Wiley from https://doi.org/10.1002/cmdc.201700141
MB (both thioester and free thiol forms) are generally unstable in nature due to hydrolysis of the thioester and oxidation of the free thiol and, hence, difficult to use as drugs. To circumvent this chemical instability, a series of prodrug forms of MB was developed by attaching methylbutyrate group to the S-atom of the MB and screened for stability and anti-HIV activity. These types of molecules are being developed as potential new treatments for HIV infection, as they show low-micromolar range anti-HIV activity in cell-based assay. The MB prodrugs were synthesized from 2-amino benzoic acid in four straightforward transformations. The anti-HIV activity for the MB and the MB prodrugs are exactly same because the MB prodrugs first form the corresponding free thiol in presence of esterase enzymes in the cell and then acetyl CoA promotes acetylation of thiol group to form the thioesters. Subsequently, acetyl transfer to the sulfur atom of cysteine in NCp7 destabilizes the zinc coordination, which leads to protein unfolding and loss of function.
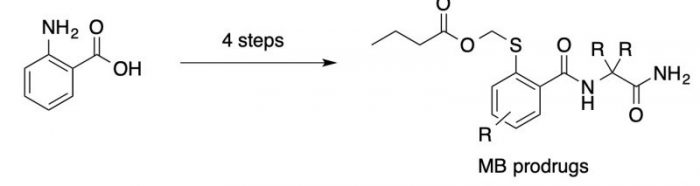
Figure 3. General structure and synthesis of MB prodrugs. Image published with permission from Wiley from https://doi.org/10.1002/cmdc.201700141
In conclusion, a series of novel 2-mercaptobenzamide prodrugs were synthesized from inexpensive commercially available starting materials. The MB prodrugs were stable at ambient temperature and exhibited relatively good antiviral activity. Further structure-activity relationship (SAR) studies with different MB prodrugs would give new avenues for anti-HIV drug discovery and development.
These findings are described in the article entitled Probing Mercaptobenzamides as HIV Inactivators via Nucleocapsid Protein 7, recently published in the journal ChemMedChem. This work was conducted by Mrinmoy Saha, Michael T. Scerba, Nathaniel I. Shank, Stewart R. Durell, and Daniel H. Appella from the National Institutes of Health, and Tracy L. Hartman, Caitlin A. Buchholz, and Robert W. Buckheit Jr. from ImQuest Biosciences.
References:
- Hartman, T. L.; Yang, L.; Helfrick, A. N.; Hassink, M.; Shank, N. I.; Rosenker, K. G.; Scerba, M. T.; Saha, M.; Hughes, E.; Wang, A.; Xu, X.; Gupta, P.; Buckheit Jr., R.W.; Appella, D. H. Antiviral Research 2016, 134, 216-225.
- Saha, M.; Scerba, M. T.; Shank, N. I.; Hartman, T. L.; Buckheit Jr., R.W.; Appella, D. H. ChemMedChem 2017, 12, 714-721.









