
Scientists are always fascinated by the various kinds of adaptations that mangroves possess to survive in coastal areas. Mangroves are salt-tolerant plants growing in tropical and subtropical coastlines (mainly between latitudes 25°N and 25°S). Although these plants constitute only 0.5% of the world’s coastal area, they provide billions of dollars in terms of ecosystem services such as fisheries, nutrient cycling, and carbon storage1-3.
If you visit any mangrove ecosystem represented by Avicennia or Sonneratia species, you will be surprised to see that these plants possess unique roots called pneumatophores. Unlike the roots of other marine and terrestrial plants, these pneumatophores emerge from the muddy sediments of the mangrove floor and grow in an upward direction (negatively geotropic). As the mangrove sediments are hypoxic, the pneumatophores possess minute pores called lenticels on their surface for the intake of oxygen from the atmosphere, and which carry out respiration. Hence, these roots are called respiratory roots, which help the host plants survive in the hypoxic mud.

Republished with permission from Elsevier from: https://doi.org/10.1016/j.ecss.2017.08.018
Although these functions of pneumatophores have been known for a long time, our study evaluated the pneumatophores of Avicennia officinalis (L.) collected from Goa (central west coast of India) from a habitat point of view. It was found that these roots are home to diverse species of microalgae and meiofauna (metazoan invertebrates and foraminifera in the size range 63-500 µm). The major microalgae were tychoplanktons (pseudoplanktons) such as Coscinodiscus sp., Thalassionema sp., Cyclotella sp., Fragilariopsis sp. and Biddulphia sp. These microalgae are planktonic genera and might have settled on pneumatophores from the water column during high tide. On the other hand, the major meiofaunal groups were nematoda, harpacticoida, diptera, halacarida, tanaidacea, and foraminifera. These meiofaunal organisms either take refuge against predators in the pneumatophore habitat or exploit the oxygen for respiration purposes during gas exchange via the pneumatophore surface.
Among the microalgae and meiofauna associated with the pneumatophores, several species were rare (abundance <1%) and unique (exclusively found in pneumatophore habitat). Although these rare and unique species are neglected in ecological studies owing to their low abundance or restricted distribution, they play multiple roles in the ecosystem:
- They, together with common (dominant) species, utilize the available resources more efficiently than the common species does alone4;
- The biomass of rare microalgae contributes to the overall primary productivity and ecosystem functioning of the mangrove forests5;
- Our study revealed the presence of oribatid mites, which enhance the soil fertility either by decomposing the organic detritus found on the pneumatophore surface or feeding on the dead pneumatophores and converting this fecal pellets that easily get assimilated in the mangrove soil6-7; and
- In addition to these roles, the rare species enhance the functional resilience of the ecosystem by forming backup for the dominant species in response to any kind of perturbations8.
Mangrove pneumatophores are home to several new species, which are unknown to science. So far, 29 new species have been reported worldwide only from the surface of mangrove roots (including pneumatophores), indicating their biodiversity potential9. We have also discovered two new species: one species of uropodina mite, Eutrachytes flagellatus, and another species of tanaidacea, Teleotanais indiaensis, from the surface of pneumatophores9-10.
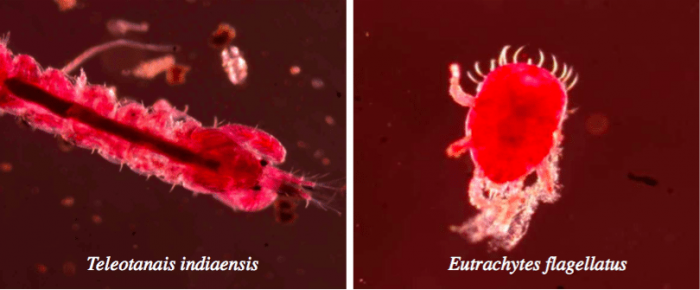
Credit: Gobardhan Sahoo
Although more studies on pneumatophore-associated organisms are yet to be carried out in some major mangrove ecosystems of India, they are currently on the verge of extinction due to two main threats: (1) Deforestation of mangrove forests (reduced canopy density) might affect the adaptability of pneumatophore-associated organisms, as direct sunlight and UV radiation might reach the pneumatophores, and (2) anthropogenic pollution as pneumatophores tend to accumulate toxic heavy metals and pesticides11-12.
The pneumatophores are host to several unique and rare species which are highly sensitive to environmental perturbations. Hence, any loss could be permanent. In this scenario, it is necessary to document these novel organisms from other mangrove ecosystems of the world and evaluate their role in the ecosystem functioning.
These findings are described in the article entitled, Epibiotic communities (microalgae and meiofauna) on the pneumatophores of Avicennia officinalis (L.), recently published in the journal Estuarine, Coastal and Shelf Science. This work was conducted by Gobardhan Sahoo, Z. A. Ansari, Jamila BiShaikh, Sandesh U. Varik, and Mangesh Gauns from the CSIR-National Institute of Oceanography, India.
References:
- Alongi, D.M. (2014). Carbon cycling and storage in mangrove forests. Annual Review of Marine Science. 6:195-219.
- Donato, D.C., et al. (2011). Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience. 4: 293-297.
- Lee, S.Y., et al. (2014). Ecological role and services of tropical mangrove ecosystems: a reassessment. Global Ecology and Biogeography. 23: 726-743.
- Loreau, M. and Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature. 412: 72-76.
- Lyons, K.G., et al. (2005). Rare species and ecosystem functioning. Conservation Biology. 19: 1019-1024.
- Siira-Pietikäinen, A., et al. (2008). Oribatid mites (Acari: Oribatida) in boreal forest floor and decaying wood. Pedobiologia. 52: 111-118.
- Syamjith, P.K. and Ramani, N. (2013). Association of a mangrove dwelling euphthiracarid mite, Acrotritia clavata (Märkel 1964) (Acari: Oribatida) with the epiphytic alga, Microspora sp. International Journal of Acarology. 39: 615-619.
- Weithoff, G. (2003). The concepts of ‘plant functional types’ and ‘functional diversity’ in lake phytoplankton –a new understanding of phytoplankton ecology. Freshwater Biology. 48: 1669-1675.
- Moraza, M.L., Kontschán, K., Sahoo, G. and Ansari, Z.A. (2016). A new species of Eutrachytes (Acari: Uropodina: Eutrachytidae) associated with the Indian mangrove (Avicennia officinalis). Acarologia. 56: 73-89.
- Larsen, K., Sahoo, G. and Ansari, Z.A. (2013). Description of a new mangrove root dwelling species of Teleotanais (Crustacea: Peracarida: Tanaidacea) from India, with a key to Teleotanaidae. Species Diversity. 18: 237-243.
- Shete, A., Gunale, V.R. and Pandit, G.G. (2007). Bioaccumulation of Zn and Pb in Avicennia marina (Forsk.) Vierh. and Sonneratia apetala Buch. Ham. from urban areas of Mumbai (Bombay), India. Journal of Applied Sciences and Environmental Management. 11: 109-112.
- Shete, A., Gunale, V.R. and Pandit, G.G. (2009). Organochlorine pesticides in Avicennia marina from the Mumbai mangroves, India. Chemosphere. 76: 1483-1485.
- Sahoo G., Ansari Z.A., Jamila Bi Shaikh, Sandesh U. Varik and Mangesh Gauns. 2018. Epibiotic communities (microalgae and meiofauna) on the pneumatophores of Avicennia officinalis (L.). Estuarine, Coastal and Shelf Science. 207: 391-401.









