
The highly polarizing topic of hydraulic fracturing, which is used to stimulate sequestered hydrocarbons from impermeable rock formations during unconventional oil and gas development, is an inherently thirsty process that can require millions of gallons of fresh water, while also producing millions of gallons of chemically rich waste.
As such, the recent expansion of unconventional oil and gas development (UD) across the western United States has brought forth major concerns regarding the widespread utilization of freshwater resources and the induction of anthropogenic earthquakes that can result during the subsurface disposal of oilfield waste.
What if we could “kill two birds with one stone” and treat the produced waste to the point that it could be reused for the stimulation of subsequent production wells? This would not only reduce the reliance on freshwater resources, but it would also reduce the occurrence of induced seismicity.
Unfortunately, waste streams from UD, commonly referred to as produced water and flowback water, can be incredibly complex matrices that are comprised of a myriad of organic, inorganic, and biological constituents, which can preclude their reuse from practically any application. For example, the presence of certain volatile organic compounds and metal ions can affect downhole polymer chemistry, whereas various species of sulfate-reducing and iron-oxidizing bacteria can cause the souring of produced hydrocarbons, as well as compromise production infrastructure. Collectively, these contaminants have traditionally rendered the repurposing of these waste fluids a significant challenge, which in turn has made the subsurface disposal of oilfield waste a more functional and convenient option.
In light of this growing environmental issue, our research team recently partnered up with industry colleagues to evaluate a wide range of water treatment technologies (ie. ozonation, particulate filtration, UV exposure, and the use of variable carbon media), to ultimately assess the feasibility of oilfield waste recycling under field conditions. Screening hundreds of samples for over 2,500 variables, we observed raw waste samples that exhibited total organic carbon levels as high as 1,500 mg/L, be treated to a clean state (<10 mg/L) resembling unperturbed brackish groundwater. Collectively, our experiments revealed that multiple treatment technologies were required in order to remove pertinent organic, inorganic, and biological contaminants below their respective reuse thresholds.
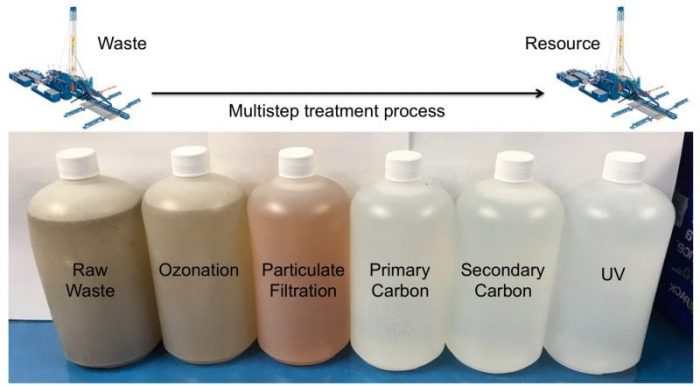
Credit: Zacariah Hildenbrand
While this study identified that a number of technologies are required to transform produced oilfield waste into a resource for production well stimulation, the next challenge will be to find additional desalination technologies that can convert this fluid into a viable source for agricultural discharge and other beneficial uses. This is particularly germane to the Permian Basin region of the American southwest where six barrels of wastewater are being produced for every barrel of oil. The increased number of production wells, producing millions of barrels of waste, coupled with the increased scrutiny surrounding injection well permitting (because they have been linked to earthquakes), has provided somewhat of a perfect storm whereby quantum amounts of waste need to be treated, desalinated, and then discarded responsibly.
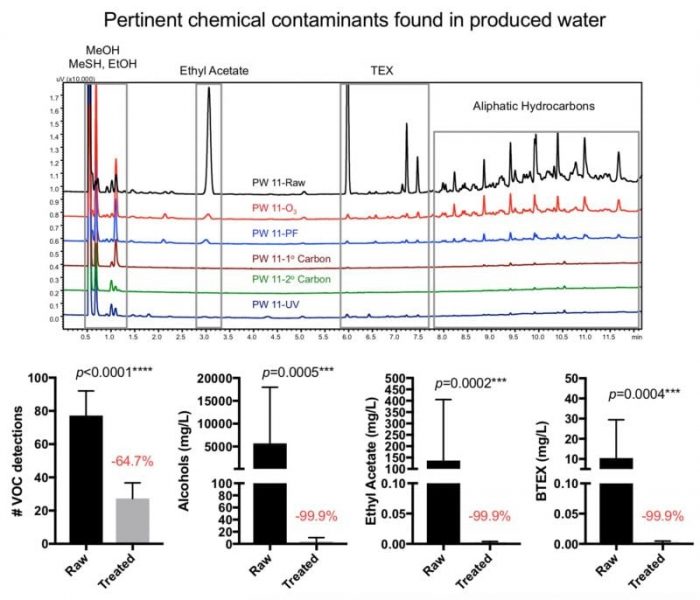
Credit: Zacariah Hildenbrand
Generally, filtration modalities such as reverse osmosis are ideal for the removal of trace metals and salts. However, oilfield wastes can exhibit salt concentrations more than four times that of seawater, rendering traditional modalities ineffective and cost-prohibitive. As such, subsequent collaborations with industry engineers provide us with the greatest opportunity to address this high stakes challenge.
These findings are described in the article entitled Characterizing variable biogeochemical changes during the treatment of produced oilfield waste, recently published in the journal Science of the Total Environment. This work was conducted by Zacariah L. Hildenbrand, Inês C. Santos, Tiffany Linden, Doug D. Carlton Jr., Emmanuel Varona-Torres, Misty S. Martin, Michelle L. Reyes, Safwan R. Mulla, and Kevin A. Schug from the Collaborative Laboratories for Environmental Analysis and Remediation (CLEAR) at the University of Texas at Arlington, in collaboration with Challenger Water Solutions, LLC.









