
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main types of an autoimmune disease called Inflammatory Bowel Disease (IBD). Since the beginning of the 21st century, IBD has become a global disease with an increasing incidence in newly-industrialized countries whose societies have become Westernized. Although in Europe and North America the incidence of the illness is stabilizing, the prevalence remains high as it exceeds 0.3%.
These data highlight the need to investigate new treatments and innovative systems to manage this complex and life-long disease. Therefore, the development of animal models of the disease could be interesting since it allows researchers to test experimental drugs, evaluate their efficacy, and choose therapies with better results for their implementation in clinical practice. Nevertheless, there is still a need for less invasive diagnostic techniques.
In clinical practice, endoscopy and biopsy are the gold standards for diagnosis and management of IBD patients, but both procedures are highly invasive and uncomfortable for patients. Unlike these traditional techniques, molecular imaging techniques provide additional data from, for example, areas that colonoscopy cannot reach, such as the small intestine, and a more transmural view not only limited to the mucosal layer.
Currently, there are many animal models available. For our study, we chose the TNBS animal model, which consists of the induction of an experimental colitis through the use of a haptenizing agent called 2,4,6-trinitrobenzenesulfonic acid. This reagent causes a T-cell mediated immune response that produces acute necrosis and transmural inflammation in the colon wall, which resembles the human disease. On the other hand, it was necessary to validate the model, so a corticoid commonly used in clinical practice (Prednisolone) was administered to a group of animals with IBD, and then compared to the group of animals without treatment.
Although molecular imaging techniques such as Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) are not currently used in clinical practice to monitor IBD patients, in vivo molecular imaging of small animals is increasingly being developed for the assessment of disease-specific animal models. Miniaturized versions of clinical devices are available for small animal imaging in preclinical research.
In our study, we used a μPET/CT (Micro- Positron Emission Tomography/Computed Tomography) to accurately follow the evolution of the illness over time. Moreover, to visualize areas of inflammation we used the radiotracer [18F]Fluoro-2-deoxy-2-D-Glucose ([18F]FDG), which identifies areas with high glucose metabolism due to inflammation or infection and, thus, there is a increase in the [18F]FDG uptake that it is represented in the PET images as areas of more intense color. The CT scan offers an anatomical reference frame to the physiological data obtained by PET through the use of a contrast agent (white color).
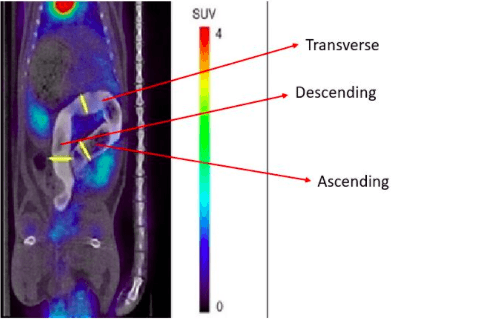
Figure: Different Regions of Interest (ROIs) at ascending, transverse and descending colon. Image courtesy Anxo Fernández-Ferreiro
Is there a positive response to the corticoid treatment?
The Standardized Uptake Value (SUVmax) is a quantification parameter that gives us data about existing inflammation in the colon. This quantitative analysis is carried out by using circularly delineated Regions of Interest (ROIs) on the images to calculate the maximum [18F]FDG uptake value. In the 3 first days after the induction of colitis, the SUVmax values of both treated and non-treated groups of animals were elevated. On day 3 after induction, the corticosteroid treatment was started, and from days 7-13. the SUVmax values were significantly decreased in the treated group but not in the non-treated ones. This shows a positive response to the corticoid treatment, given that lower SUVmax values mean that there is a remission in the colonic inflammation.
All these results were also checked by the histologic analysis of colon tissues obtained from the sacrificed animals. The samples were evaluated by a digestive pathologist using a microscope and the disease activity was scored in five different grades using the Nancy histological index, with Grade 0 being the absence of significant histological disease and Grade 4 the ulceration of colonic mucosa with inflamed granulation tissue.
The results from the histologic analysis support those obtained in the PET studies, showing significant associations between SUVmax and Nancy grades, which are validated indexes to assess disease activity in IBD patients. The positive response obtained with the corticosteroid treatment and the parameters used to assess remission not only validate the animal model, but also highlight its translational value.
What conclusion can be drawn from this study?
The knowledge obtained from the use of animal models in preclinical research is large, and there is no doubt about their contribution in defining the pathogenesis of IBD. They help to understand the complex interactions between the gut microbiota and the host, and how genetic and environmental factors could affect homeostasis. In combination with molecular imaging, it is possible to characterize the disease in a non-invasive way, allowing repeated investigations on each living animal, thereby limiting the number of animals needed during the follow-up.
In summary, the TNBS animal model assessed by PET/CT has a potential utility in preclinical research, since it resembles the human disease and therefore, can be used in the discovery process of new drugs and in the evaluation of the therapeutic activity of experimental compounds.
These findings are described in the article entitled Longitudinal PET/CT evaluation of TNBS-induced inflammatory bowel disease rat model, recently published in the International Journal of Pharmaceutics.
This work was conducted by Iria Seoane-Viaño, Asteria Luzardo-Álvarez, and Francisco Otero-Espinar from the Universidade de Santiago de Compostela (USC), Noemí Gómez-Lado and Jesús Silva-Rodríguez from the Universidade de Santiago de Compostela and Health Research Institute of Santiago de Compostela (IDIS), Héctor Lázare-Iglesias, Manuel Barreiro-de Acosta, and José Ramón Antúnez-López from the University Clinical Hospital Santiago de Compostela, Michel Herranz from the Health Research Institute of Santiago de Compostela, María Jesús Lamas and Anxo Fernández-Ferreiro from the University Clinical Hospital Santiago de Compostela and the Health Research Institute of Santiago de Compostela, Pablo Aguiar from the Universidade de Santiago de Compostela, Health Research Institute of Santiago de Compostela, and University Clinical Hospital Santiago de Compostela, and Álvaro Ruibal from the Universidade de Santiago de Compostela, Health Research Institute of Santiago de Compostela, University Clinical Hospital Santiago de Compostela, and Tejerina Foundation.









