
Malaria is caused by mosquito-borne parasites of the genus Plasmodium, which affects over 216 million people worldwide. It is still a huge problem in developing countries because there is no good vaccine for malaria, and antimalarial drugs are losing their efficacy with drug resistance on the rise, especially in Africa and South-east Asia. In 2017 alone, there were 445,000 malaria deaths globally.
The body’s first line of defense against malaria is the innate immune system, and one of the earliest cells to respond and kill the parasites are the natural killer (NK) cells. The outcome of this early response can determine whether severe malaria will occur. Interestingly, the response of NK cells towards malaria parasite is not the same in everyone — NK cells from some individuals kill more parasites (responsive), while those from others kill less (non-responsive) — and no one knows why this happens. When we compare the NK cells from malaria patients with severe disease against those with mild disease, we observe that NK cells from patients with severe disease were less responsive than NK cells from patients with mild disease. This spurred us to ask why NK cells from different individuals respond to malaria differently.
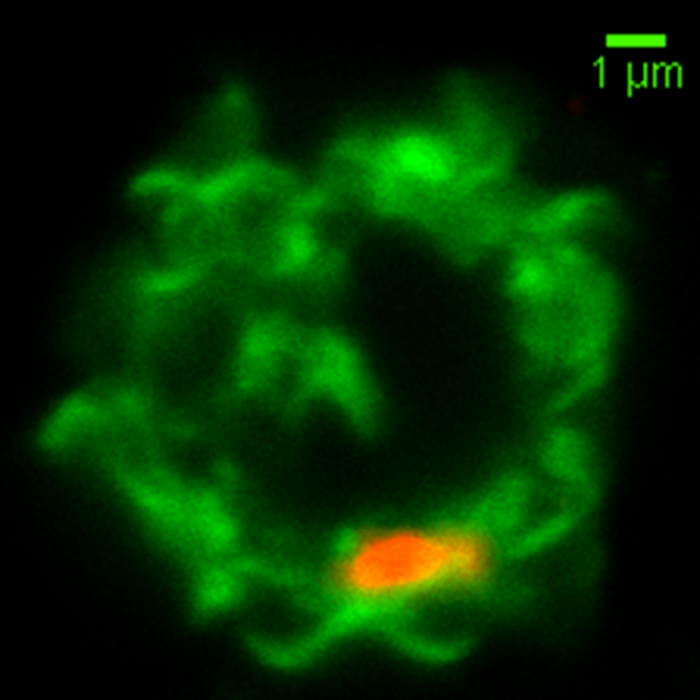
This is an image of a NK cell membrane (labeled in green) fusing with a red colored malaria-microvesicle. Image credit: Weijian Ye.
By comparing responsive and non-responsive NK cells, we found that when exposed to malaria parasites, responsive NK cells have a higher expression of a molecule, MDA5, compared to non-responsive NK cells. MDA5 is a receptor that detects foreign nucleic acid, and in response to it, MDA5 activates NK cells. MDA5 is classically associated with viral infections — when a virus infects a cell, it brings along its viral nucleic acids into the cell, thus exposing them to MDA5 recognition. However, in the case of malaria, which doesn’t infect NK cells, things are not so direct.
So, how does MDA5 detect malaria nucleic acids when malaria doesn’t infect NK cells? We found that NK cells fuse with malaria-derived membranous microvesicles, which have diameters under one micrometer. These microvesicles contain malaria nucleic acids and are secreted by malaria-infected cells, serving as a form of cell-to-cell communication. Via the exchange of nucleic acids, drug resistance genes can be passed between malaria-infected cells. On this note, the fusing of NK cells with microvesicles releases malaria nucleic acids into the NK cell, thereby triggering MDA5. MDA5 then activates NK cells, and cause them to go into a “high-alert” state. This increases the ability of NK cells in surveying its surrounding, leading to the improved killing of malaria-infected cells.
Having found the signals required to trigger MDA5, we then packaged synthetic nucleic acids into membranous microvesicles and asked if these synthetic microvesicles can help to improve the response of non-responsive NK cells. Indeed, when we expose non-responsive NK cells to these synthetic microvesicles in addition to malaria-infected cells, we observe that the non-responsive NK cells now kill better, reaching almost the same malaria-killing efficiency as responsive NK cells. This represents a new paradigm for the treatment of infectious diseases such as malaria. In contrast to traditional anti-malaria drugs which target the parasite itself, our new treatment targets cells of the body’s immune system. This prevents the generation of drug resistance, as there is no drug-pressure directed at the parasite. In addition, as the treatment increases the responsiveness of the body’s immune system against foreign pathogens, it is also possible to utilize similar immune-modulating drugs against other infectious diseases.

From left to right: Authors Marvin Chew, Weijian Ye and Corresponding author Prof. Peter Preiser. Image courtesy Weijian Ye.









