
Biochar is a carbon-rich product that comes from biomass pyrolysis. Biochar has sparked a wide interest due to its great potential for (i) improving soil fertility, (ii) enhancing carbon sequestration, and (iii) favoring soil remediation.
The application of biochar mainly implies surface functional groups, especially for the immobilization of toxic metals (not heavy metals; see box below).
For example, as reviewed by Pourret and Houben (2018), biochar may bind aluminum (Al), predominantly due to complexation of Al with the hydroxyl and carboxyl group at the surface of biochar particles. Likewise, biochar may sorb copper (Cu) and lead (Pb), using their oxygen-containing carboxyl, hydroxyl, and phenolic functional surface groups. It is, therefore, necessary to understand biochar’s surface reactivity to its application.
Analytical approaches have been frequently employed to characterize the functional groups of biochar; they include Fourier Transform Infrared Spectroscopy (FTIR), or Nuclear Magnetic Resonance Spectroscopy. Yet, these techniques do not allow scientists to determine to what extent each functional group contributes to metal binding under various conditions. Earlier research on the organic material has displayed that metal binding to carboxylic and phenolic sites was strongly dependent on the pH and ionic strength in the aqueous phase.

The non-scientific term “heavy metal” is no longer used; experts instead give the name of the considered elements or name them as a group, such as metals and metalloids (for further explanation see the Pourret and Bollinger, 2018).
Pourret, O. and Bollinger, J.-C. (2018) “Heavy metal” – What to do now: To use or not to use? Sci. Total Environ. 610, 419-420. Credit: David Houben
Characterization of these organic materials has also highlighted that carboxylic groups commonly display low metal affinity sites, while phenolic groups have high-affinity ones. Understanding the behavior and the relative contribution of each type of groups (i.e., carboxylic and phenolic) to metal binding by biochar under various conditions (i.e., pH and ionic strengths) is therefore crucial (i) to gain insight into the metal binding with biochar and (ii) to predict metal behavior with biochar over a wide range of conditions.
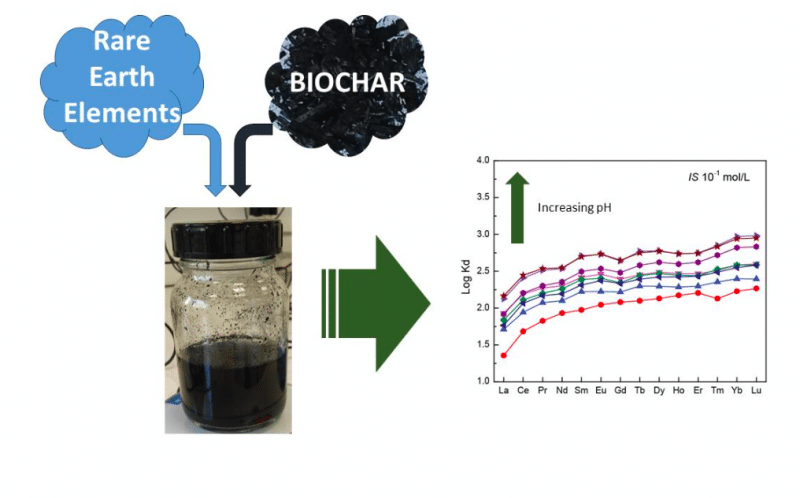
Analyzing the relative distribution (hereafter described as a pattern) of rare earth element (REE) sorption by organic compounds has become an emergent approach for characterizing the surface reactivity of these organic substances. In this study, we only consider the fourteen stable lanthanides (from La to Lu) as the rare earth elements that form a group presenting very similar and coherent chemical properties. Due to their specific properties, REE partition coefficients (used to estimate potential dissolved metal sorption by a solid phase like biochar) between organic material and aqueous solutions are extremely sensitive to the binding sites’ heterogeneity (i.e. functional group nature).
Specific partition coefficient patterns arise, depending on the type of the major binding sites at the organic substance’s surface. For example, a log partition coefficient pattern exhibits a medium REE (MREE) downward concavity when REE binding occurs at carboxylic sites. Conversely, REE binding to phenolic groups results in a log partition coefficient pattern exhibiting a regular increase from La to Lu. These results allow the use of REEs as a tracer to determine which of the carboxylic, phenolic or chelate functional groups are implicated in metal binding to organic material under various environmental conditions.
Pourret and Houben’s study (2018) developed this new approach based on previous works on REE interactions with organic matter to better understand the nature and the role of metal binding sites to biochar under various pH and ionic strengths.
Credit: Olivier Pourret
Rare earth elements sorption onto biochar was analyzed using batch experiments (from pH 3 to 9 and IS 10-1 mol/L to 10-3 mol/L). Rare earth element patterns exhibited a Middle REE (MREE) downward concavity at pH below 5 and ionic strength representative of low salinity conditions (i.e. 10-3 mol/L). These REE patterns are in agreement with current datasets quantifying REE binding with organic material. Definitely, the MREE downward concavity exhibited by REE-biochar pattern matches REE patterns with various organic substances (e.g. humic acids, acetate, etc.).
Thanks to such similarities in comparing pattern shapes, it helps us highlight the fact that carboxylic groups are the main REE-binding sites in biochar. Eventually, the metal bonding strength with biochar increases when pH and ionic strength increase. It suggests that biochar is more efficient for long-term metal immobilization at circumneutral pH and relatively high ionic strength. These results were further verified by using speciation modeling, and conclusions were confirmed.
Unlike techniques such as FTIR, which give no detail on the biochar surface reactivity behavior, our present contribution based on previous works on REE-organic material interactions was the first struggling to explain the relative distribution of metal binding sites onto biochar under various conditions (i.e. various pH and ionic strengths). Our results specify that environmental conditions affect not only the quantity of sorbed metal onto biochar but also the metal-biochar binding strengths and stabilities. In more details, the strength of the metal binding with biochar increases when pH and ionic strength increase. Indeed, such results has a great implication for the use of biochar as a metal sorbent in the long run. Our study also highlights the great potential of REEs as a fingerprint for the characterization of metal binding sites onto sorbing matrixes, including biochar.
These findings are described in the article entitled Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint, recently published in the journal Heliyon. This work was conducted by Olivier Pourret and David Houben from UniLaSalle.









