Crystallization is a cheap and relatively straightforward way of purifying materials. It’s not surprising that it’s a technique of choice for many industries, including the pharmaceutical industry where purity can be critical. It might be surprising, then, to learn that crystallization is not always a good thing.
There are many ways in which crystallization is not desirable; for example, you may end up crystallizing a solid that you didn’t want or you may end up crystallizing on surfaces, causing blockages. This second type of undesirable crystallization is called scaling or scale formation. Most industrial processes have to deal with scale, but, in particular, solids that have low solubilities are difficult to manage (because their low solubility means you don’t need much of them before crystallization happens).
The most common scales are calcium sulfate dihydrate (known as gypsum), calcium carbonate, and barium sulfate. Out of these three solids, the one with the lowest solubility is barium sulfate. Barium sulfate scale is common in off-shore oil production. Seawater has sulfate in it, and oil/gas reservoirs often also have water in them, known as aquifer water. When the two mix, the barium ions in the aquifer water join with the sulfate ions in seawater and form barium sulfate. In order to avoid this from happening, additives that impact the process of crystallization can be used. The additive can act on the ions in the solution (known as chelating agents), binding them and limiting the amount of solids that form. However, the best additives are those that are called “threshold inhibitors.” These additives are effective at controlling crystallization at extremely low doses.
My research is all about understanding how impurities impact crystallization processes and how we can predict their behavior. Once we know this, we can develop appropriate strategies to limit scale formation. In this paper, we are looking at how a molecule called oxalate interacts with barium sulfate crystallization. In the case of calcium carbonate, oxalate forms a layer of a solid called calcium oxalate, and this stops more calcium carbonate from forming on the solid’s surface. Oxalate can bind barium ions in the solution, but is this how it impacts? So, we thought it would be a good idea to see what happens in the case of barium sulfate.
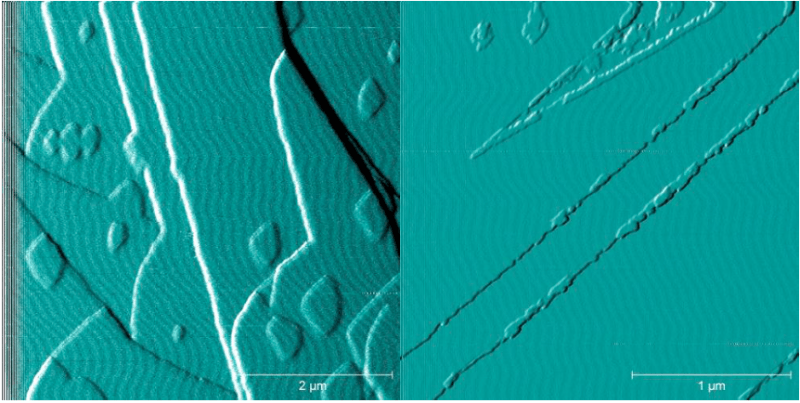
What we learned is that oxalate does not form a new, different layer on the barium sulfate surface, but it clearly does stick to the surface. Amazingly, we can follow this process as it happens in solution using an atomic force microscope. The presence of oxalate stops the crystal surface of barium sulfate from growing.
In Figure 1a you can see how the surface normally grows; with new “islands” of barium sulfate sprouting and growing, but in Figure 1b, there are much fewer islands — the surface is almost perfectly flat. So, oxalate is able to inhibit crystallization but not by impacting on the ions in solution (chelation). Ultimately, oxalate has an impact similar to a threshold inhibitor and acts in a similar way to many threshold inhibitors — it sticks to sites that are critical to crystal growth.
These findings are described in the article entitled The impact of oxalate ions on barium sulfate crystallization, recently published in the Journal of Crystal Growth. This work was conducted by Franca Jones, Mark I. Ogden, and Tomoko Radomirovic from Curtin University.









