
Ever since flowering plants appeared on Earth around the Early Cretaceous (between 130 and 90 million years ago) (1), they have evolved and diversified through very complex biogeographical processes (2). Some of those were particular to a set of lineages, while others were mainly widespread.
Researchers, however, can now only see the actual scenario based on knowledge that has been vastly documented in museums and herbaria. And here is what we’ve got so far: different sets of plant species are somehow “spatially structured,” forming an outstanding mosaic of species distribution. Biogeography then emerged, mainly in the 19th century, as a field of biological sciences which tackles such distributional puzzles. Therefore, by considering a comparative and classificatory perspective, biogeographers have tried to describe past, present, and future spatial patterns, to achieve a full comprehension of the processes that have, has, and will generate those patterns.
Considering only plant species (but for animals the story is quite similar), we know there are six major biogeographical realms, each one with a unique set of floristic composition (3). The Neotropical region, whose boundaries almost correspond exactly to Latin America, except for some portions in southern South America and northern Mexico, has the vast majority of species in the globe: there are at least 40,000 flowering plants unique – in other words, endemic – to the area (4). Brazil, one of the largest countries of the Western World and the largest in Latin America, houses the major slice of this enormous diversity: there are more than 33,000 species of flowering plants occurring in this country in different forested or savanna-like biomes (5).
The geobiotic scenario in the Neotropics is also complex. Many things happened in the past few million years: the Andes uplift, the rise of the Panama Isthmus, changes to the largest drainage system in the world in terms of flow volume – the Amazon River (6); and the replacement of the ancient South American tropical rainforests corridor – formed by the Amazon and Atlantic Forests – by open, seasonally dry vegetation (7). Such geobiological events have obviously affected the local fauna and flora, sometimes in different manners, but sometimes in such a way that events can reveal a unique shared history.
In the case of tropical rainforests, the focus of our survey, we believe some particular events, which we shall address henceforth, have contributed to the biota distribution we have documented so far. Still, this is only possible due to patterns that were once identified and further described. Thus, setting the identity of particular bioregions of the world involves describing and properly classifying those new areas in coherent classification systems, as well as describing new methods that can estimate those regions with lots of endemic species. As a matter of fact, we can name those bioregions as “areas of endemism” (8).
That said, we performed a survey of biogeographical pattern recognition using flowering plants of the Rutaceae group — the orange, grapefruit and lime family — as a study model. This group is particularly diverse in the Neotropics, with many species centered in forested areas where we focused our pattern study. So, we selected one tribe of this family, the Galipeinae (Fig. 1). The Galipeinae subtribe has 28 genera and c. 130 species described, and it is endemic to the Neotropics. Most of their representatives are narrow endemic to particular portions of the Atlantic Forest, but also to the Amazon and even to some open seasonally dry vegetations, such as the Brazilian Cerrado. Hence, we advocate that this group can serve as a great model to bring novel insights to the biogeography and evolution of tropical rainforests, as well as to the natural history of Galipeinae itself.

Figure 1. A general panorama at the morphological diversity of the Galipeinae. (A-C) (A to C) Fruit and flower details of Hortia brasiliana Vand. ex DC.; (D) Conchocarpus heterophyllus (A.St.-Hil.) Kallunki & Pirani; (E) Conchocarpus cyrtanthus Kallunki; (F) Erythrochiton brasiliensis Nees & Mart.; (G) Galipea ciliata Taub.; (H) Sigmatanthus trifoliatus Huber ex Emmerich; (I) Rauia nodosa (Engl.) Kallunki; (J) capsules of Neoraputia trifoliata (Engl.) Emmerich ex Kallunki; (K) Spiranthera odoratissima A.St.-Hil.; (L) Neoraputia magnifica (Engl.) Emmerich ex Kallunki. Photos by: H. Moreira – A, B; F. Obermuller – C; J.H.L. El-Ottra – D, H, L; J.R. Pirani – E; D.G. Almeida-Costa – F; I. Cordeiro – G; P. Dias – I; A. Popovkin – J; J. Jardim – K. Reprinted from Flora, 251, Colli-Silva, Pirani, Biogeographic patterns of Galipeinae (Galipeeae, Rutaceae) in Brazil: Species richness and endemism at different latitudes of the Atlantic Forest “hotspot”, 77-87, 2019, with permission from Elsevier.
We then proceeded to perform a manual extensive revision of all distribution data of the Galipeinae species. Through a large evaluation of available literature and revisitation of the specimens deposited in the herbaria and museums, we attributed, corrected, or selected geographical coordinates for each occurrence record and mapped their distribution. Then, each distribution map was assembled, and we checked the spots in Brazil where the distribution pattern of two or more endemic species were alike. Those were later defined as the so-called “areas of endemism” of the group on the study area.
In summary, we found three “hot spots” of occurrence of endemic species for the Galipeinae in the Atlantic Forest (Fig. 2). In other words, the Galipeinae species are not randomly distributed through different latitudes of the Brazilian eastern coast, and we have several endemic species restricted to these three portions instead. Interestingly, those identified areas of endemism were also documented for other groups in other studies for both plants and animals (harvestmen, birds, squamates). The question is: how could we explain the converging distribution in these three spots of Atlantic Forest?
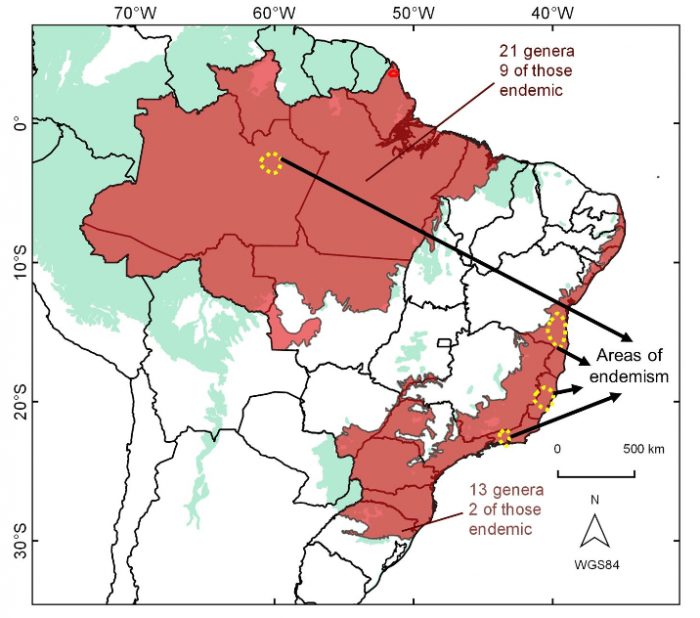
Figure 2. Summary of the distribution data and analyses of the subtribe Galipeinae in tropical rainforests, shaded in red (Amazon more in the left, Atlantic Forest in the right). Most of the areas of endemism are centered in different latitudes of the coastal Brazilian Atlantic Forest. Reprinted from Flora, 251, Colli-Silva, Pirani, Biogeographic patterns of Galipeinae (Galipeeae, Rutaceae) in Brazil: Species richness and endemism at different latitudes of the Atlantic Forest “hotspot”, 77-87, 2019, with permission from Elsevier.
This is actually a tough question, but we basically discuss in our paper two classical hypotheses. The first is the forest refugia hypothesis (6, 9). It states that, since the late Pleistocene (between 126 to 11.6 thousand years ago), climatic fluctuations caused continuous forest contractions and retractions. In other words, during drier and colder periods, the distribution of forest organisms that depends on humidity would have been narrowed, and savanna-like vegetations would be able to expand instead.
Conversely, during humid and warmer periods, the wettest areas would again expand, reconnecting the isolated “refuges” of evergreen forests that were once disrupted. Such “evergreen forest refugia” would be therefore spots that could split the local fauna and flora. Thus, several continuous populations would be isolated for a while, accumulating several mutations and evolving, until, when they eventually reconnected, you could distinguish two new separate lineages: one sister to the other, each one narrow endemic to a particular spot of the forest. We name such natural phenomena as “vicariant speciation,” and each refugium would play a role as “vicariant agent” in the speciation process of lineages that were once contiguous.
A very handy hypothesis indeed, except we have compiled some refuting evidence for so. Several new biological data for particular groups, as well new geological evidence for South America, have shown that the age of most forest lineages would be much older than the late Pleistocene, where such fluctuations would have happened (10). So, alternative hypotheses have emerged, and new vicariant agents of speciation are being suggested. Large and wide rivers are one of those, and they might play a role especially for the Amazon lineages, given the significant historical variations in the drainage system of the Amazon River (6). For the Atlantic Forest, another alternative, even more thought-provoking hypothesis has been proposed: the “Atlantis Forest Hypothesis” (11). This hypothesis states that sea periodical regressions and transgressions in the Atlantic Ocean flooded different portions of the Atlantic Forest, splitting lineages into “islands” surrounded by sea. This idea, however, also has received criticism (12).
In the end, we still cannot say for sure what happened with the Galipeinae – and maybe we never will; as a matter of fact, one alternative scenario is that all these hypotheses may have acted together, as they are not exclusive. But with only distribution data and what we already know from the literature, take a look at what we already can do and hypothesize. We can now move straightforward in downstream analyses that incorporate new biological (phylogenetic) and geological (climatic, edaphic) data, for example. Now that we have these three main areas of endemism recovered for the Galipeinae, the next step is to test each candidate hypothesis (or perhaps even propose a new scenario?) towards a fuller panorama of the geobiotic history of South America and of different groups of flowering plants.
These findings are described in the article entitled Biogeographic patterns of Galipeinae (Galipeeae, Rutaceae) in Brazil: Species richness and endemism at different latitudes of the Atlantic Forest “hotspot”, recently published in the journal Flora.
References:
- Crane, P.R., Friis, E.M. & Pedersen, K.R. (1995) The origin and early diversification of angiosperms. Nature, 374, 27-33.
- Antonelli, A., Ariza, M., Albert, J., Andermann, T., Azevedo, T., Bacon, C., Faurby, S., … & Edwards, S.V. (2018) Conceptual and empirical advances in Neotropical biodiversity research. PeerJ, 6, e5644.
- Morrone, J.J. (2015) Biogeographical regionalization of the world: a reappraisal. Australian Systematic Botany, 28, 81-90.
- Ulloa-Ulloa, C., Acevedo-Rodrígues, P., Beck, S., Belgrano, M.J., Bernal, R., Berry, P.E., Brako, L., … & Jørgensen, P.M. (2017) An integrated assessment of the vascular plant species of the Americas. Science, 358(6370): 1614-1617.
- Flora do Brasil 2020 under construction. Jardim Botânico do Rio de Janeiro. Available at: <http://floradobrasil.jbrj.gov.br/>. Accessed on: 24 May 2019.
- Antonelli, A. & Sanmartín, I. (2011) Why are there so many plant species in the Neotropics? Taxon, 60, 403-414.
- Thode, V.A., Sanmartín, I. & Lohmann, L.G. (2019) Contrasting patterns of diversification between Amazonian and Atlantic Forest clades of Neotropical lianas (Anphilophium, Bignonieae) inferred from plastid genomic data.
- Morrone, J.J. (2018) The spectre of biogeographical regionalization. Journal of Biogeography, 45, 282-288.
- Bush, M.B. & Oliveira, P.E. (2006) The rise and fall of the Refugial Hypothesis of Amazonian speciation: a paleoecological perspective. Biota Neotropica, 6, 1-17.
- Leite, Y.L.R., Costa, L.P., Loss, A.C., Rocha, R.G., Batalha-Filho, H., Bastos, A.C., … & Pardini, R. (2016) Neotropical forest expansion during the last glacial period challenges refuge hypothesis. PNAS, 113, 1008-1013.
- Amaral, F.R., Edwards, S.V., Pie, M.R., Jennings, W.B., Svensson-Coelho, M., d’Horta, F.M., Schmitt, C.J. & Maldonado-Coelho, M. (2016) The “Atlantis Forest hypothesis” does not explain Atlantic Forest phylogeography. PNAS, 113, E2097-E2098.









