
Lighting our towns and cities, the vehicles we drive, and even charging and powering electronic devices all depend on energy from fuel. As the world transitions away from traditional fossil fuel sources, there is a need for sustainable “green” fuel sources to provide energy.
Hydrogen (H2) has emerged as one of the most promising sustainable fuels: it has a high energy density (120 MJ/kg) and burns cleanly, producing only water and heat as byproducts. However, H2 does not exist naturally on earth, and most hydrogen is produced by natural gas reforming, which is largely seen as an unsustainable long-term production method. As the sun is one of the earth’s indefinite resources, it is unsurprising that solar hydrogen production is a desirable path forward.
Solar assisted water splitting (also known as photoelectrochemical water splitting) is the process of using the sun’s energy and a catalyst to break water (H2O) into hydrogen and oxygen. This water splitting reaction produces no greenhouse gasses or dangerous byproducts and could provide a pathway for sustainable hydrogen production. Of many photoactive catalysts, the iron oxide crystal known as hematite is one of the most attractive materials for studying this reaction. It is able to absorb light across a large part of the solar spectrum, giving it a theoretical solar to hydrogen conversion efficiency of 15%. Additionally, it is inexpensive, abundant, non-toxic, and well positioned to perform the tricky oxygen-evolution half-reaction. However, hematite underperforms experimentally compared to its promise, largely due to its poor electronic properties such as high rates of charge recombination and sluggish reaction rates at its surface.
Is it possible to modify the surface of hematite and improve its ability to perform the water-splitting reaction? Yes. In fact, several researchers have demonstrated that inorganic and organic surface coatings have this effect. Specifically, carbon coatings have shown great potential for increasing hematite’s performance (measured by the value of its photocurrent density). But the mechanism by which these carbon coatings improve hematite is poorly understood. In our recent work, we propose and study the effect of one atomic layer of carbon (graphene) as an overlayer on hematite in an attempt to uncover this mechanism.
Graphene has properties unique from other types of carbon that make it a good potential overlayer to both improve a photoelectrode and study the mechanism behind this improvement. Its high mobility charge carriers may help offset hematite’s poor electrical properties; it is inert and therefore stable in the water-splitting environment. Single-layer graphene can be reliably synthesized via a chemical vapor deposition method and easily transferred onto the surface of a photocatalyst, allowing for easy and reproducible fabrication.
The graphene coating does improve hematite’s ability to catalyze the water-splitting reaction: the photocurrent density increases 1.6 times with good reproducibility in the presence of graphene compared to the bare photoelectrode.
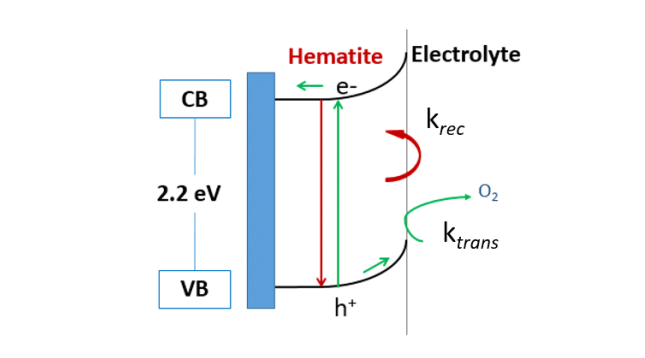
Figure 1. Band diagram of hematite depicting charge separation and transfer (green arrows), charge recombination (red arrows). Image courtesy Melissa Cardona.
To further explore the role of the graphene overlayer, kinetic studies are performed. The overall efficiency of the reaction is dependent on two rate constants: how fast charges transfer from the surface into the water-splitting reaction (rate of charge transfer, ktrans) and how quickly charges recombine (krec) (depicted in Figure 1). We found that the rate of charge transfer actually decreases in the presence of graphene. As for the rate of charge recombination, a dramatic reduction is observed.
For example, at a given potential, the charges on the surface of the bare hematite studied here recombine at a rate of 71 sec-1 while the graphene-coated hematite has a recombination rate of 13 sec-1. This substantial decrease in recombination results in a higher yield of charge carriers to participate in the water-splitting reaction, which is likely an underlying cause of the improved performance. Overall, this increases the charge transfer efficiency of the reaction by 8%.
In conclusion, a graphene-coated hematite photoelectrode can be made by transferring single-layer graphene to the surface of hematite, which demonstrates higher efficiency of the water-splitting half-reaction. While hematite was the test-material chosen for this study, we believe that graphene and other carbon-based overlayers may be useful for reducing surface recombination and improving the catalytic properties of a range of semiconductor photoelectrodes. Understanding the water-splitting reaction and mechanisms to improve it is essential for ultimately designing high efficiency, commercially viable photoelectrodes and photoelectrochemical cells for sustainable production of hydrogen fuel.
These findings are described in the article entitled The role of graphene as an overlayer on nanostructured hematite photoanodes for improved solar water oxidation, recently published in the journal Materials Today Energy.









