
Rare earth elements (REE) have become a highly valuable commodity due to their increased use in emerging technological applications. The future exploitation of areas with high REE content will play a strategic geopolitical role around the world, as shown in a previous article.
The progressing activity in opening new mining areas will contribute to increased REE mobility and will influence the fate of these elements in the environment over the next decades. In order to monitor the respective contribution of mining activity, it is crucial to establish a toxicity threshold in these areas.
Moreover, the need for coal is decreasing, and the industry is under fire for polluting streams and rivers with coal ash and acid mine drainage (AMD) as shown in this previous article. Among all the metals potentially present in coal and released into the environment by AMD, REE are now being looked at more closely. If one can figure out how to extract them economically, REE sales could help pay for part of the cleanup costs associated with the coal mining industry.
However, coal mining depletes and contaminates water resources, leaving previously cultivable land unsuitable for agriculture. Coal mining activities thus reduce available ground- and stream-water and pollute these vital resources with AMD and released metals (including REE). Populations living near coal mines have to use mine pit water for culture irrigation.
In a recently published study, Martinez and co-workers tried (1) to quantify REE effect on rice plant growth and (2) to determine whether iron (III) oxide presence on plant root surface (i.e. iron oxide plaques) played a role in inhibiting toxic effects caused by REE occurrence. Rice plant growth was performed in a greenhouse under controlled hydroponic conditions. Plants were exposed to REE, and to iron (II) sulfate or iron (II) chloride (Figure 1).
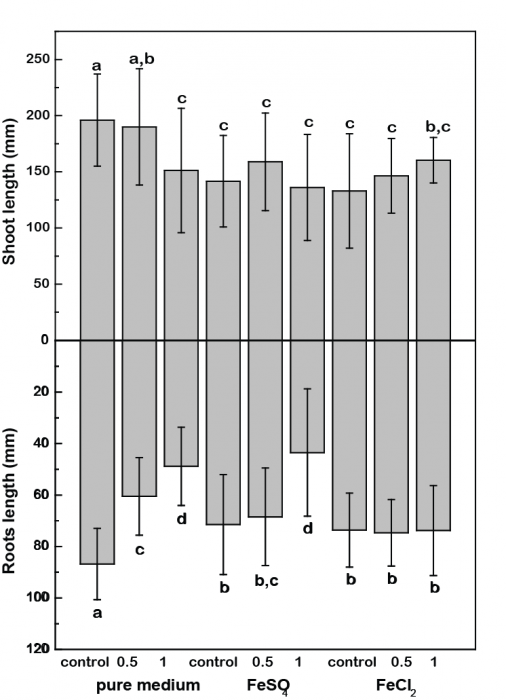
Figure 1. Effects of REE on rice shoot and root biomass. Credit: Olivier Pourret
In the presence of the Fe(II) sulfate, a negative growth effect was observed, which was more noticeable at the highest REE concentrations.
For the Fe(II) chloride experiments, speciation modeling calculated that REEs are present as hydrated ions (REE3+) or sorbed by Fe(III) oxyhydroxides. With Fe(II) chloride, the light rare earth elements (La, Ce, Pr, Nd; LREE) remained mostly soluble, whereas the middle (Sm, Eu, Gd; MREE) and heavy (Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu; HREE) rare earth elements were mostly bound by Fe(III) oxide surfaces. As negative growth effects were observed with Fe(II) chloride, the most soluble LREE could be settled to play a role in the inhibition of rice plant growth.
Furthermore, upon addition of Fe(II) sulfate, the MREE and HREE were significantly complexed by sulfate. It results in a regained toxic effect for rice plants, especially at highest REE concentration. It also put forward the effect of dissolved MREE- and HREE- sulfate complexes on plant growth. This observation, coupled with the knowledge that sulfate is an essential nutrient for plants, suggests an absorption of these species by rice plants.
This study results strongly evidence that REEs are harmful to rice development. These negative growth effects may have been attenuated as a consequence of REE sorption onto iron oxide plaques observed on the root surface. This hypothesis was further suggested by surface complexation modeling of REE to iron (III) oxides.
These findings are described in the article entitled Effect of rare earth elements on rice plant growth, recently published in the journal, Chemical Geology. This work was conducted by Raul E. Martinez and Charlotte Dian from Albert-Ludwigs University and Olivier Pourret and Michel-Pierre Faucon from UniLaSalle.









