The issue of microplastic pollution is raising serious concern among scientists and the society at large 1,2. Microplastics, tiny plastic fragments ranging from 1 micron to 5 millimetres in size, have found their way into a variety of terrestrial and aquatic environments 3,4, marine organisms 5and even consumer products, including food 6–8and drink 9.
Are microplastics a real threat?
The risks posed by microplastics exposure are the subject of ongoing debate 10, since the possible adverse effects are dose and size dependent, with some arguing that, at present, the low concentration of microplastics in the ecosystem is likely safe and does not warrant immediate action 11. However, the actual level of exposure might be greater than current estimates lead us to believe 12, as those estimates are based on studies which routinely neglect a large population of microplastics: the very small ones.
Very small microplastics (1 – 50 microns) are potentially the most dangerous since they have a higher chance of penetrating the gut barrier 13. According to a recent review 14, a third of microplastic identification studies relies on visual inspection alone to detect whether a particle is plastic or from natural origin, a strategy that progressively fails as particle size diminishes and it is not recommended for particles smaller than 500 microns. Fortunately, almost half of the studies employ infrared spectroscopy, a chemical identification technique capable of determining the polymeric composition of particles no smaller than 15-20 microns. Yet, accounting for microplastics smaller than 20 microns is proving to be crucial.
Raman won’t let small microplastics slip under the radar
Raman spectroscopy, an alternative to FTIR, offers spatial resolution down to 1 micron and, when coupled to an optical microscope (Raman microscopy), is the only recommended method for the identification of microplastics under 20 microns in size 3.Raman analysis of microplastics found in seawater 15and freshwater 16report that more than half of sampled particles are smaller than 40 microns and their distribution follows a power law, where the population grows rapidly as size decreases (Fig 1). Another sobering example is the work of Darena Schymanski and co-workers 9, who analyzed microplastics present in bottled water, finding that 80% of those belong to the < 20 micron category – and would be entirely missed by a FTIR analysis. It is then clear that Raman techniques are necessary for achieving representative estimates of microplastic concentration, yet at present merely 13% of microplastic identification studies employ Raman microscopy14.
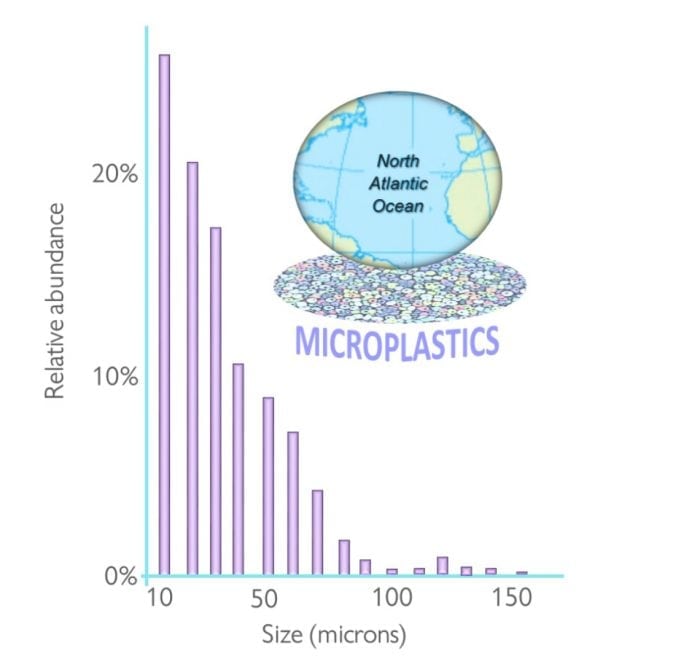
Fig 1 – Size distribution of microplastics found in the Atlantic Ocean, as reported by Enders et al., 2015 (Ref 15)
Room for improvement
FTIR and Raman share many positive features – both are relatively cheap, non-destructive and require a small amount of sample. Despite its higher spatial resolution, Raman remains unpopular, relative to FTIR, partly due to its poor signal quality 17. On one hand, the Raman signal is inherently weak, since only a tiny fraction (10-8) of photons bombarding the sample are actually translated into the Raman signal. Therefore, a good quality Raman spectrum requires longer analysis time than a FTIR spectrum of the same sample. On top of that, fluorescence stemming from organic impurities, colouring agents and other additives results in a raised spectrum baseline which often overshadows the Raman signals of the underlying polymer preventing its identification. In order to overcome these drawbacks and render Raman microscopy more attractive for large scale use, researchers in the microplastic identification field have proposed a variety of interesting solutions, as summarized in a recent review 18.
Rising above the noise
A common procedure for reducing fluorescence stemming from organic impurities (and some organic dyes) is removing these in a digestion step (with acid, base, oxidative agents…), prior to analysis 19,20. However, digestion does not remove all fluorescence-causing agents commonly encountered in microplastics. For those cases, a clever solution might be the procedure suggested by Ghosal and colleagues 21, who use an automated algorithm to remove the fluorescence background and reveal the underlying polymer spectrum, as exemplified in Fig 2. As for the inherent weakness of the Raman signal, a possible solution is the use of a detector equipped with an electron multiplier, which enhances the signal prior to readout thereby reducing the time necessary to achieve a decent signal to noise ratio 22.
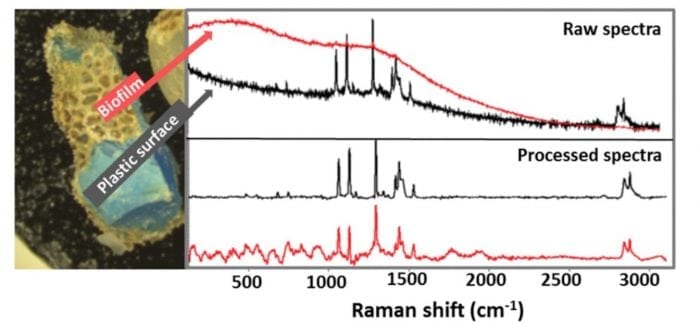
Fig 2 – The upper panel shows unprocessed Raman spectra of a plastic particle partially covered with biofilm. The characteristic Raman signals of the polymer are obvious in the spectrum collected from an uncovered portion of the plastic particle (upper panel, black line) however, due to fluorescence interference, those same signals are unidentifiable in the spectrum collected from another location where the plastic is covered in biofilm (upper panel, red line) which. After automated background removal it is possible to identify the polymer under the biofilm as polypropylene (lower panel, red line). Reprinted from Water Research, 142, C.F. Araujo, M.M. Nolasco, A.M.P. Ribeiro, P.J.A. Ribeiro-Claro, Identification of microplastics using Raman spectroscopy: Latest developments and future prospects, pp 426-440, Copyright (2018), with permission from Elsevier.
Let the machines do the dirty work
Before large-scale monitoring of microplastics using micro-Raman (or micro-FTIR) becomes a reality, a great challenge must be overcome: how to deal with the large volume of particles to be analysed and the resulting number of spectra, which need to be individually compared with reference spectra until a match is found? An obvious answer is automation. In a fully automated analysis a filter containing microplastics (along with non-plastic material) is placed under the microscope, then an image processing software locates the particles and a motorized stage places the selected particle under the Raman laser. The spectrum of the sample is collected and quickly compared against a library of reference spectra from commercial polymers until a good enough match is found and the particle is identified – all the while the operator is free to do other tasks. Following a similar strategy, Laura Frère and co-workers 23analysed 110 particles in less than 3 hours, and successfully identified 75% of those (71% were indeed of plastic nature while 4% were inorganic particles). The remainder 25% could not be identified due to the interference of fluorescence, the presence of pigments whose spectrum masks that of the polymer or inability to find a spectral match within the reference database.
Expanding the database
For automated spectral identification (library matching) to be successful, the reference library must be comprehensive, including not only the spectra of pristine polymers but also those of common plastic additives, colouring agents, fillers, etc. Ideally, the database will also include reference spectra of microplastics found in the environment whose polymeric chains are often degraded by weathering processes resulting in spectra that may differ greatly from that of the pristine polymer. Unfortunately, commercial libraries do not include reference spectra from weathered microplastics so that researchers need to collect their own and manually add them to a custom made database 24. This process is prohibitively time consuming and a smart way of distributing the load and avoiding repetition would be to create an open source database of Raman spectra. A project of this kind already exists for mass spectroscopy in the form of Curatr 25, a web application where authorized curators can upload their spectra, which are then converted into a format compatible with library matching software so that any user can freely download and use them – an idea that the Raman community may wish to adopt.
Future pathways for microplastic identification
Even though there is still plenty of room for optimization, the inherently weak signal of conventional (spontaneous) Raman spectroscopy is a great impediment to its implementation in ultra-fast, real-time monitoring of flowing microplastics. Such endeavour requires a faster technique and the answer may lie in stimulated Raman spectroscopy (SRS). SRS uses two laser beams focused on the sample to generate a signal that is orders of magnitude higher than the spontaneous Raman signal – therefore acquisition time is greatly reduced 26. Liron Zada and colleagues 27were the first to apply SRS to the detection of environmental microplastics (Fig. 3). As a proof of principle, the authors scanned 1 cm2of filter area, without any particle pre-selection, using SRS achieving a total analysis time of 4.5 hours while the same scanning procedure using spontaneous Raman scattering would take 116 days. Combining automated particle search and SRS one could significantly speed up the process.
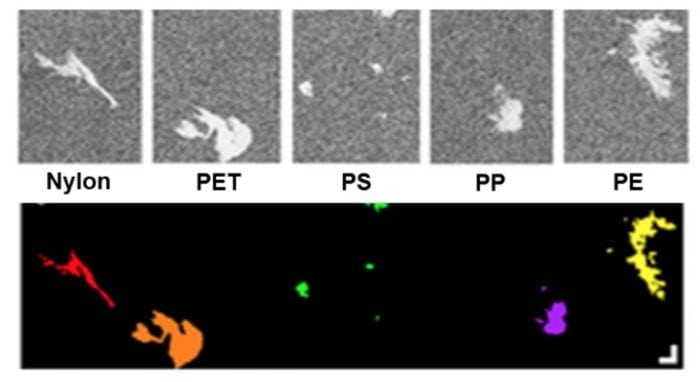
Fig. 3 – Use of Stimulated Raman Scattering for identifying microplastics from the Rhine estuary. The upper panel shows an SRS identification image, where white regions denote particles whose polymeric composition has been identified. The lower panel shows a color-coded version of the same image, with different colors distinguishing the various polymers found: nylon – red, PET (polyethylene terephthalate) – orange, PS (polystyrene) – green, PP (polypropylene) – purple and PE (polyethylene) – yellow. Adapted from the work of Zada et al., 2018 (Ref27) under a Creative Commons license (https://creativecommons. org/licenses/by/4.0/legalcode)
Another promising avenue of research is combining flow-cytometry with SRS for the detection of fast moving particles, as done by Chi Zhang and co-workers 28, whose custom-built signal amplifier allows the collection of an SRS spectrum in merely 5 microseconds. The flow-cytometry-SRS apparatus discriminated among polystyrene and poly(methyl methacrylate) beads (10 microns) flowing in water at a rate of 11 000 beads per second. Another interesting example of real time analysis is the handheld SRS microscope proposed by Liao and colleagues 29, which permits imaging at a rate of 8 frames per second.
As Raman techniques become faster, more sensitive and capable of high-throughput processing their implementation in large scale, real time monitoring of microplastics is likely to become a reality.
These findings are described in the article entitled Identification of microplastics using Raman spectroscopy: Latest developments and future prospects, recently published in the journal Water Research. This work was conducted by Catarina F. Araujo, Mariela M. Nolasco, Antonio M.P. Ribeiro, and Paulo J.A. Ribeiro-Claro from the Universidade de Aveiro.
References:
- Avio, C. G.; Gorbi, S.; Regoli, F. Plastics and Microplastics in the Oceans: From Emerging Pollutants to Emerged Threat. Blue Growth Mar. Environ. Saf. 2017, 128, 2–11.
- Horton, A. A.; Walton, A.; Spurgeon, D. J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141.
- Ivleva, N. P.; Wiesheu, A. C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem.-Int. Ed. 2017, 56 (7), 1720–1739.
- Lots, F. A. E.; Behrens, P.; Vijver, M. G.; Horton, A. A.; Bosker, T. A Large-Scale Investigation of Microplastic Contamination: Abundance and Characteristics of Microplastics in European Beach Sediment. Mar. Pollut. Bull. 2017, 123 (1–2), 219–226.
- Lusher, A. L.; Welden, N. A.; Sobral, P.; Cole, M. Sampling, Isolating and Identifying Microplastics Ingested by Fish and Invertebrates. Anal. Methods 2017, 9 (9), 1346–1360.
- Karami, A.; Golieskardi, A.; Choo, C. K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and Mesoplastic Contamination in Canned Sardines and Sprats. Sci. Total Environ. 2018, 612, 1380–1386.
- Karami, A.; Golieskardi, A.; Choo, C. K.; Larat, V.; Galloway, T. S.; Salamatinia, B. The Presence of Microplastics in Commercial Salts from Different Countries. Sci. Rep. 2017, 7, 46173.
- Karami, A.; Golieskardi, A.; Ho, Y. B.; Larat, V.; Salamatinia, B. Microplastics in Eviscerated Flesh and Excised Organs of Dried Fish. Sci. Rep. 2017, 7, 5473.
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of Microplastics in Water by Micro-Raman Spectroscopy: Release of Plastic Particles from Different Packaging into Mineral Water. Water Res. 2018, 129 (Supplement C), 154–162.
- Backhaus, T.; Wagner, M. Microplastics in the Environment: Much Ado about Nothing? A Debate. PeerJ Prepr. 2018, 6, e26507v6.
- Burton, G. A. Stressor Exposures Determine Risk: So, Why Do Fellow Scientists Continue To Focus on Superficial Microplastics Risk? Environ. Sci. Technol. 2017, 51 (23), 13515–13516.
- Conkle, J. L.; Del Valle, C. D. B.; Turner, J. W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manage. 2018, 61 (1), 1–8.
- Alexander, J.; Barregard, L.; Bignami, M.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; et al. Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. Efsa J. 2016, 14 (6), UNSP 4501.
- Renner, G.; Schmidt, T. C.; Schram, J. Analytical Methodologies for Monitoring Micro(Nano)Plastics: Which Are Fit for Purpose? Micro Nanoplastics Ed. Dr Teresa AP Rocha-St. 2018, 1, 55–61.
- Enders, K.; Lenz, R.; Stedmon, C. A.; Nielsen, T. G. Abundance, Size and Polymer Composition of Marine Microplastics >= 10 Mu m in the Atlantic Ocean and Their Modelled Vertical Distribution. Mar. Pollut. Bull. 2015, 100 (1), 70–81.
- Erni-Cassola, G.; Gibson, M. I.; Thompson, R. C.; Christie-Oleza, J. A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 Mm to 20 Mu m) in Environmental Samples. Environ. Sci. Technol. 2017, 51 (23), 13641–13648.
- Ribeiro-Claro, P.; Nolasco, M. M.; Araújo, C. Chapter 5 – Characterization of Microplastics by Raman Spectroscopy. In Comprehensive Analytical Chemistry; Rocha-Santos, T. A. P., Duarte, A. C., Eds.; Characterization and Analysis of Microplastics; Elsevier, 2017; Vol. 75, pp 119–151.
- Araujo, C. F.; Nolasco, M. M.; Ribeiro, A. M. P.; Ribeiro-Claro, P. J. A. Identification of Microplastics Using Raman Spectroscopy: Latest Developments and Future Prospects. Water Res. 2018, 142, 426–440.
- Miller, M. E.; Kroon, F. J.; Motti, C. A. Recovering Microplastics from Marine Samples: A Review of Current Practices. Mar. Pollut. Bull. 2017, 123 (1–2), 6–18.
- Silva, A. B.; Bastos, A. S.; Justino, C. I. L.; da Costa, J. P.; Duarte, A. C.; Rocha-Santos, T. A. P. Microplastics in the Environment: Challenges in Analytical Chemistry – A Review. Anal. Chim. Acta 2018.
- Ghosal, S.; Chen, M.; Wagner, J.; Wang, Z.-M.; Wall, S. Molecular Identification of Polymers and Anthropogenic Particles Extracted from Oceanic Water and Fish Stomach – A Raman Micro-Spectroscopy Study. Environ. Pollut. 2018, 233, 1113–1124.
- Dieing, T.; Hollricher, O. High-Resolution, High-Speed Confocal Raman Imaging. Vib. Spectrosc. 2008, 48 (1), 22–27.
- Frere, L.; Paul-Pont, I.; Moreau, J.; Soudant, P.; Lambert, C.; Huvet, A.; Rinnert, E. A Semi-Automated Raman Micro-Spectroscopy Method for Morphological and Chemical Characterizations of Microplastic Litter. Mar. Pollut. Bull. 2016, 113 (1–2), 461–468.
- Lenz, R.; Enders, K.; Stedmon, C. A.; Mackenzie, D. M. A.; Nielsen, T. G. A Critical Assessment of Visual Identification of Marine Microplastic Using Raman Spectroscopy for Analysis Improvement. Mar. Pollut. Bull. 2015, 100 (1), 82–91.
- Palmer, A.; Phapale, P.; Fay, D.; Alexandrov, T. Curatr: A Web Application for Creating, Curating and Sharing a Mass Spectral Library. Bioinformatics 2017, btx786–btx786.
- Min, W.; Freudiger, C. W.; Lu, S.; Xie, X. S. Coherent Nonlinear Optical Imaging: Beyond Fluorescence Microscopy. Annu. Rev. Phys. Chem. 2011, 62 (1), 507–530.
- Zada, L.; Leslie, H. A.; Vethaak, A. D.; Tinnevelt, G.; Janssen, J.; Boer, J. F. de; Ariese, F. Fast Microplastics Identification with Stimulated Raman Scattering Microscopy. J. Raman Spectrosc. 2018.
- Zhang, C.; Huang, K.-C.; Rajwa, B.; Li, J.; Yang, S.; Lin, H.; Liao, C.; Eakins, G.; Kuang, S.; Patsekin, V.; et al. Stimulated Raman Scattering Flow Cytometry for Label-Free Single-Particle Analysis. Optica 2017, 4 (1), 103–109.
- Liao, C.-S.; Wang, P.; Huang, C. Y.; Lin, P.; Eakins, G.; Bentley, R. T.; Liang, R.; Cheng, J.-X. In Vivo and in Situ Spectroscopic Imaging by a Handheld Stimulated Raman Scattering Microscope. ACS Photonics 2017.









