
Spontaneous coronary artery dissection (SCAD), defined as non-traumatic, non-iatrogenic dissociation of the coronary vessel wall, represents an important cause of acute coronary syndrome (ACS) among young or middle-aged women without conventional risk factors for atherosclerosis. While the true incidence of SCAD remains unclear due to the under-diagnosis, SCAD has been detected in 2 to 4% of the angiograms performed in patients with ACS and is considered as the most common etiology of ACS in pregnant women and women ≤50 years of age.
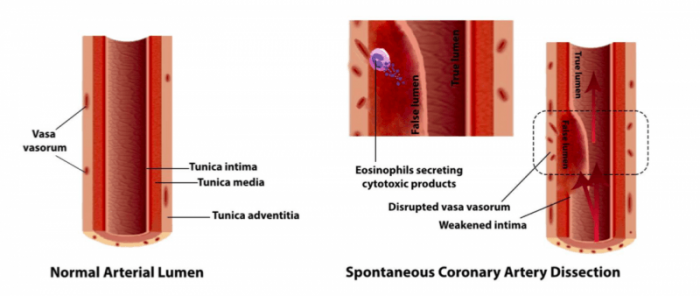
The exact pathophysiology of SCAD is still unclear. The spontaneous development of coronary dissection (separation of coronary vessel layers) has been postulated to occur from two processes: the “inside-out” and “outside-in” model. The inside-out model denotes the development of a breach or tear of the inner layer, allowing the entry of blood in the middle layer and the formation of a false lumen.
In the outside-in model, there is a direct disruption of microvessels supplying the vessel wall (termed vasa vasorum), which results in the formation of hemorrhage into the middle layer. In either model, with the accumulation of blood primarily in the middle layer of the coronary vessel, the false lumen may expand in its volume and extend axially, leading to compression of the true lumen and the occurrence of ACS.
Interestingly, there are frequent postmortem reports of eosinophilic inflammation among SCAD cases, pointing to a possible mechanistic association of eosinophil and SCAD. The work led by Dr. Gerald Chi (Beth Israel Deaconess Medical Center, Harvard Medical School) discusses the potential therapeutic role of anti-inflammatory therapy targeting eosinophils in SCAD based on three major premises.
First, histologically, SCAD is characterized by damage to the vascular endothelium and myocytes in the coronary wall, which may be caused by cytotoxic products released from eosinophils in response to inflammatory mediators. Lytic substances released by eosinophils could injure myocytes, type I and III collagen, and elastic tissue of smooth muscle present in the media, leading to disorganization and fragmentation of elastic fibers and collagen.
“Focal weakening and disruption of the intima could be mediated by the effect of eosinophil lytic granular substances, which manifests as intimal tear via the inside-out mechanism,” illustrated by the co-author Dr. Sadaf Sharfaei (Beth Israel Deaconess Medical Center, Harvard Medical School).
Credit: Sadaf Sharfaei, M.D.
Second, the development of intramural hemorrhage (the outside-in model) could also be linked to eosinophilic inflammation. Previous studies have suggested that pro-angiogenetic properties of eosinophils may be associated with neovascularization of vasa vasorum and dilatation of intimal capillaries. The newly-formed, fragile vasa vasorum is subject to disruption due to high intraluminal pressure from the interconnected capillary network under the influence of pro-angiogenetic signals from the eosinophils.
Last, eosinophils may also play a role in pregnancy-related SCAD. Alterations in estrogen and progesterone associated with uterine involution may activate eosinophils and subsequent degranulation of hydrolytic substances such as collagenases. This mechanism could explain, at least in part, the preferential occurrence of SCAD among pregnant women.
It has been estimated that approximately 15 to 30% of SCAD patients experience recurrent attacks of dissection despite conservative therapy. The optimal management strategy for SCAD recurrence remains unsettled. Thus far, there are no randomized controlled trials evaluating the safety and efficacy pharmacological treatment such as antiplatelet agents, beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins. More importantly, none of these agents target the potential mechanistic role of eosinophilic inflammation frequently reported in SCAD.
A preliminary report indicates that therapy with prednisone and cyclophosphamide may promote healing of dissected segments and attenuation of further vascular wall insult by inhibiting periadventitial inflammation mediated by eosinophils. Indeed, immunosuppressive therapy could be a feasible approach, particularly for recurrent episodes involving multiple vessels that are surgically inoperable. Additionally, eosinophilic infiltration has also been recognized as a contributor to late-onset coronary stent malposition, which may increase the long-term risk for stent thrombosis. In this regard, SCAD patients revascularized with stent implantation could also benefit from anti-inflammatory therapy directed against eosinophils.
The rationale of preventive therapy for SCAD recurrence is to facilitate healing of dissected coronary segments by regulating the inflammatory mediators that lead to accumulation, survival, and degranulation of eosinophils. Future research is warranted to examine the potential benefit of this anti-inflammatory strategy in reducing SCAD recurrence. Several caveats should be considered. First, it is prudent to avoid high-dose corticosteroids in the acute setting of ACS due to the possible risk of ventricular rupture. Second, eosinophilia (elevated eosinophils in the circulation) may not accurately reflect the extent of localized eosinophilic infiltration observed in SCAD. Treatment efficacy may be better demonstrated by alleviation of cardiac symptoms and resolution of dissection on angiography.
These findings are described in the article entitled Eosinophilic inflammation in spontaneous coronary artery dissection: A potential therapeutic target?, recently published in the journal Medical Hypotheses. This work was conducted by Anmol Pitliya, Sudarshana Datta, Arzu Kalayci, Farima Kahe, Sadaf Sharfaei, Mehrian Jafarizade, Sogand Goudarzi, and Gerald Chi from the Beth Israel Deaconess Medical Center, Harvard Medical School.









